Distinct mucosal endotypes as initiators and drivers of rheumatoid arthritis
- PMID: 39251771
- PMCID: PMC12573308
- DOI: 10.1038/s41584-024-01154-0
Distinct mucosal endotypes as initiators and drivers of rheumatoid arthritis
Abstract
Rheumatoid arthritis (RA) is a potentially devastating autoimmune disease. The great majority of patients with RA are seropositive for anti-citrullinated protein antibodies (ACPAs), rheumatoid factors, or other autoantibodies. The onset of clinically apparent inflammatory arthritis meeting classification criteria (clinical RA) is preceded by ACPA seropositivity for an average of 3-5 years, a period that is designated as 'at-risk' of RA for ACPA-positive individuals who do not display signs of arthritis, or 'pre-RA' for individuals who are known to have progressed to developing clinical RA. Prior studies of individuals at-risk of RA have associated pulmonary mucosal inflammation with local production of ACPAs and rheumatoid factors, leading to development of the 'mucosal origins hypothesis'. Recent work now suggests the presence of multiple distinct mucosal site-specific mechanisms that drive RA evolution. Indicatively, subsets of individuals at-risk of RA and patients with RA harbour a faecal bacterial strain that has exhibited arthritogenic activity in animal models and that favours T helper 17 (TH17) cell responses in patients. Periodontal inflammation and oral microbiota have also been suggested to promote the development of arthritis through breaches in the mucosal barrier. Herein, we argue that mucosal sites and their associated microbial strains can contribute to RA evolution via distinct pathogenic mechanisms, which can be considered causal mucosal endotypes. Future therapies instituted for prevention in the at-risk period, or, perhaps, during clinical RA as therapeutics for active arthritis, will possibly have to address these individual mechanisms as part of precision medicine approaches.
© 2024. Springer Nature Limited.
Figures

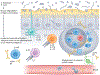

References
-
- Lee DM & Weinblatt ME Rheumatoid arthritis. Lancet 359, 903–911 (2001). - PubMed
-
- Gravallese EM & Firestein GS Rheumatoid arthritis — common origins, divergent mechanisms. N. Engl. J. Med 388, 529–542 (2023). - PubMed
-
- Karlson EW & Costenbader KH Epidemiology: interpreting studies of interactions between RA risk factors. Nat. Rev. Rheum 6, 72–73 (2010). - PubMed
-
- Silman AJ Epidemiology of rheumatoid arthritis. APMIS 102, 721–728 (1994). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

