Oligodendroglial fatty acid metabolism as a central nervous system energy reserve
- PMID: 39251890
- PMCID: PMC11452346
- DOI: 10.1038/s41593-024-01749-6
Oligodendroglial fatty acid metabolism as a central nervous system energy reserve
Erratum in
-
Author Correction: Oligodendroglial fatty acid metabolism as a central nervous system energy reserve.Nat Neurosci. 2024 Oct;27(10):2046. doi: 10.1038/s41593-024-01776-3. Nat Neurosci. 2024. PMID: 39289565 Free PMC article. No abstract available.
Abstract
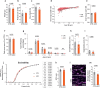
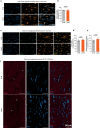
Brain function requires a constant supply of glucose. However, the brain has no known energy stores, except for glycogen granules in astrocytes. In the present study, we report that continuous oligodendroglial lipid metabolism provides an energy reserve in white matter tracts. In the isolated optic nerve from young adult mice of both sexes, oligodendrocytes survive glucose deprivation better than astrocytes. Under low glucose, both axonal ATP levels and action potentials become dependent on fatty acid β-oxidation. Importantly, ongoing oligodendroglial lipid degradation feeds rapidly into white matter energy metabolism. Although not supporting high-frequency spiking, fatty acid β-oxidation in mitochondria and oligodendroglial peroxisomes protects axons from conduction blocks when glucose is limiting. Disruption of the glucose transporter GLUT1 expression in oligodendrocytes of adult mice perturbs myelin homeostasis in vivo and causes gradual demyelination without behavioral signs. This further suggests that the imbalance of myelin synthesis and degradation can underlie myelin thinning in aging and disease.
© 2024. The Author(s).
Conflict of interest statement
A.B. is cofounder and shareholder of Casma Therapeutics and an advisory board member of Avilar Therapeutics and Amplify Therapeutics. The other authors declare no competing interests.
Figures



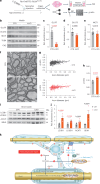
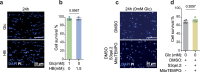
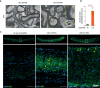
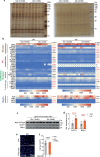



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

