Evaluation of the Fill-it-up-design to use historical control data in randomized clinical trials with two arm parallel group design
- PMID: 39251907
- PMCID: PMC11382532
- DOI: 10.1186/s12874-024-02306-2
Evaluation of the Fill-it-up-design to use historical control data in randomized clinical trials with two arm parallel group design
Abstract
Purpose: In the context of clinical research, there is an increasing need for new study designs that help to incorporate already available data. With the help of historical controls, the existing information can be utilized to support the new study design, but of course, inclusion also carries the risk of bias in the study results.
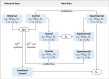
Methods: To combine historical and randomized controls we investigate the Fill-it-up-design, which in the first step checks the comparability of the historical and randomized controls performing an equivalence pre-test. If equivalence is confirmed, the historical control data will be included in the new RCT. If equivalence cannot be confirmed, the historical controls will not be considered at all and the randomization of the original study will be extended. We are investigating the performance of this study design in terms of type I error rate and power.
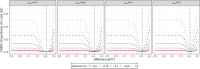
Results: We demonstrate how many patients need to be recruited in each of the two steps in the Fill-it-up-design and show that the family wise error rate of the design is kept at 5 . The maximum sample size of the Fill-it-up-design is larger than that of the single-stage design without historical controls and increases as the heterogeneity between the historical controls and the concurrent controls increases.
Conclusion: The two-stage Fill-it-up-design represents a frequentist method for including historical control data for various study designs. As the maximum sample size of the design is larger, a robust prior belief is essential for its use. The design should therefore be seen as a way out in exceptional situations where a hybrid design is considered necessary.
Keywords: Equivalence; External controls; Historical control; Power; Randomized clinical trial; Sample size; Type I error probability.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Dynamic use of historical controls in clinical trials for rare disease research: A re-evaluation of the MILES trial.Clin Trials. 2023 Jun;20(3):223-234. doi: 10.1177/17407745231158906. Epub 2023 Mar 17. Clin Trials. 2023. PMID: 36927115 Free PMC article.
-
Design of randomized controlled confirmatory trials using historical control data to augment sample size for concurrent controls.J Biopharm Stat. 2019;29(3):558-573. doi: 10.1080/10543406.2018.1559853. Epub 2019 Jan 6. J Biopharm Stat. 2019. PMID: 30612514
-
A novel equivalence probability weighted power prior for using historical control data in an adaptive clinical trial design: A comparison to standard methods.Pharm Stat. 2021 May;20(3):462-484. doi: 10.1002/pst.2088. Epub 2021 Jan 20. Pharm Stat. 2021. PMID: 33474798 Free PMC article.
-
Randomized phase II designs.Clin Cancer Res. 2009 Mar 15;15(6):1883-90. doi: 10.1158/1078-0432.CCR-08-2031. Epub 2009 Mar 10. Clin Cancer Res. 2009. PMID: 19276275 Free PMC article. Review.
-
Limitations of randomized clinical trials. Proposed alternative designs.Clin Chem Lab Med. 2000 Dec;38(12):1217-23. doi: 10.1515/CCLM.2000.192. Clin Chem Lab Med. 2000. PMID: 11205684 Review.
References
-
- Committee for Medicinal Products for Human Use (CHMP). Guideline on clinical trials in small populations. European Medicines Agency. 2006; CHMP/EWP/83561/2005. https://www.ema.europa.eu/en/clinical-trials-small-populations-scientifi....
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

