Phenotypic and spatial heterogeneity of CD8+ tumour infiltrating lymphocytes
- PMID: 39251981
- PMCID: PMC11382426
- DOI: 10.1186/s12943-024-02104-w
Phenotypic and spatial heterogeneity of CD8+ tumour infiltrating lymphocytes
Abstract
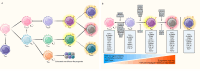
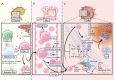
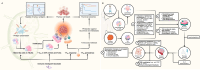
CD8+ T cells are the workhorses executing adaptive anti-tumour response, and targets of various cancer immunotherapies. Latest advances have unearthed the sheer heterogeneity of CD8+ tumour infiltrating lymphocytes, and made it increasingly clear that the bulk of the endogenous and therapeutically induced tumour-suppressive momentum hinges on a particular selection of CD8+ T cells with advantageous attributes, namely the memory and stem-like exhausted subsets. A scrutiny of the contemporary perception of CD8+ T cells in cancer and the subgroups of interest along with the factors arbitrating their infiltration contextures, presented herein, may serve as the groundwork for future endeavours to probe further into the regulatory networks underlying their differentiation and migration, and optimise T cell-based immunotherapies accordingly.
Keywords: CD8+ T cells; Cancer immunotherapy.; Caner immunotypes; T cell exclusion; T cell nomenclature; Tumour-infiltrating lymphocytes.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Grants and funding
- 82273178/National Natural Science Foundation of China
- 82273178/National Natural Science Foundation of China
- 82273178/National Natural Science Foundation of China
- 82273178/National Natural Science Foundation of China
- 82273178/National Natural Science Foundation of China
- 82273178/National Natural Science Foundation of China
- 82273178/National Natural Science Foundation of China
- 82273178/National Natural Science Foundation of China
- 82273178/National Natural Science Foundation of China
- 82273178/National Natural Science Foundation of China
- 82273178/National Natural Science Foundation of China
- 82273178/National Natural Science Foundation of China
- 82273178/National Natural Science Foundation of China
- 2023ZR108/Zhejiang Province Traditional Chinese Medicine Science and Technology Plan
- 2023ZR108/Zhejiang Province Traditional Chinese Medicine Science and Technology Plan
- 2023ZR108/Zhejiang Province Traditional Chinese Medicine Science and Technology Plan
- 2023ZR108/Zhejiang Province Traditional Chinese Medicine Science and Technology Plan
- 2023ZR108/Zhejiang Province Traditional Chinese Medicine Science and Technology Plan
- 2023ZR108/Zhejiang Province Traditional Chinese Medicine Science and Technology Plan
- 2023ZR108/Zhejiang Province Traditional Chinese Medicine Science and Technology Plan
- 2023ZR108/Zhejiang Province Traditional Chinese Medicine Science and Technology Plan
- 2023ZR108/Zhejiang Province Traditional Chinese Medicine Science and Technology Plan
- 2023ZR108/Zhejiang Province Traditional Chinese Medicine Science and Technology Plan
- 2023ZR108/Zhejiang Province Traditional Chinese Medicine Science and Technology Plan
- 2023ZR108/Zhejiang Province Traditional Chinese Medicine Science and Technology Plan
- 2023ZR108/Zhejiang Province Traditional Chinese Medicine Science and Technology Plan
LinkOut - more resources
Full Text Sources
Medical
Research Materials

