MiRNA-132/212 encapsulated by adipose tissue-derived exosomes worsen atherosclerosis progression
- PMID: 39252021
- PMCID: PMC11386123
- DOI: 10.1186/s12933-024-02404-x
MiRNA-132/212 encapsulated by adipose tissue-derived exosomes worsen atherosclerosis progression
Abstract
Background: Visceral adipose tissue in individuals with obesity is an independent cardiovascular risk indicator. However, it remains unclear whether adipose tissue influences common cardiovascular diseases, such as atherosclerosis, through its secreted exosomes.
Methods: The exosomes secreted by adipose tissue from diet-induced obesity mice were isolated to examine their impact on the progression of atherosclerosis and the associated mechanism. Endothelial apoptosis and the proliferation and migration of vascular smooth muscle cells (VSMCs) within the atherosclerotic plaque were evaluated. Statistical significance was analyzed using GraphPad Prism 9.0 with appropriate statistical tests.
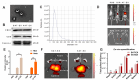
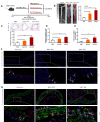
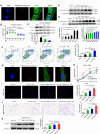
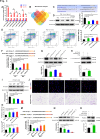
Results: We demonstrate that adipose tissue-derived exosomes (AT-EX) exacerbate atherosclerosis progression by promoting endothelial apoptosis, proliferation, and migration of VSMCs within the plaque in vivo. MicroRNA-132/212 (miR-132/212) was detected within AT-EX cargo. Mechanistically, miR-132/212-enriched AT-EX exacerbates palmitate acid-induced endothelial apoptosis via targeting G protein subunit alpha 12 and enhances platelet-derived growth factor type BB-induced VSMC proliferation and migration by targeting phosphatase and tensin homolog in vitro. Importantly, melatonin decreases exosomal miR-132/212 levels, thereby mitigating the pro-atherosclerotic impact of AT-EX.
Conclusion: These data uncover the pathological mechanism by which adipose tissue-derived exosomes regulate the progression of atherosclerosis and identify miR-132/212 as potential diagnostic and therapeutic targets for atherosclerosis.
Keywords: Adipose tissue; Atherosclerosis; Melatonin; MiR-132/212; Obesity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
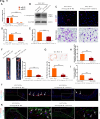
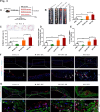
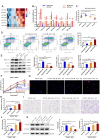
References
-
- Hiuge-Shimizu A, Kishida K, Funahashi T, Ishizaka Y, Oka R, Okada M, Suzuki S, Takaya N, Nakagawa T, Fukui T, Fukuda H, Watanabe N, Yoshizumi T, Nakamura T, Matsuzawa Y, Yamakado M, Shimomura I. Absolute value of visceral fat area measured on computed tomography scans and obesity-related cardiovascular risk factors in large-scale Japanese general population (the VACATION-J study). Ann Med. 2012;44:82–92. 10.3109/07853890.2010.526138 - DOI - PubMed
-
- Neeland IJ, Ross R, Despres JP, Matsuzawa Y, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FB, Griffin B, Zambon A, Barter P, Fruchart JC, Eckel RH. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7:715–25. 10.1016/S2213-8587(19)30084-1 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- W20243019/Health Research Project of Hunan Provincial Health Commission
- 2022105330213/National Undergraduate Innovation Training Program of Central South University
- 2022105330235/National Undergraduate Innovation Training Program of Central South University
- No. 20231696/The Health Research Project in Hunan Province
- 202103062278/The Health Research Project in Hunan Province
- 82200869/National Natural Science Foundation of China
- 82100494/National Natural Science Foundation of China
- No. 82370892/National Natural Science Foundation of China
- 82070910/National Natural Science Foundation of China
- No. 2022JJ40721/the Natural Science Foundation of Hunan Province
- 2022JJ40715/the Natural Science Foundation of Hunan Province
- No. 2021YFC2501701/National Key Research and Development Program of China
- Z2023026/National Clinical Key Specialties Major Research Projects
LinkOut - more resources
Full Text Sources
Medical
Research Materials

