Aqueous extractable nonfibrillar and sarkosyl extractable fibrillar Alzheimer's disease tau seeds have distinct properties
- PMID: 39252090
- PMCID: PMC11382398
- DOI: 10.1186/s40478-024-01849-1
Aqueous extractable nonfibrillar and sarkosyl extractable fibrillar Alzheimer's disease tau seeds have distinct properties
Abstract
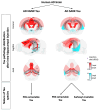
Pathological tau fibrils in progressive supranuclear palsy, frontotemporal dementia, chronic traumatic encephalopathy, and Alzheimer's disease each have unique conformations, and post-translational modifications that correlate with unique disease characteristics. However, within Alzheimer's disease (AD), both fibrillar (sarkosyl insoluble (AD SARK tau)), and nonfibrillar (aqueous extractable high molecular weight (AD HMW tau)) preparations have been suggested to be seed-competent. We now explore if these preparations are similar or distinct in their in vivo seeding characteristics. Using an in vivo amplification and time-course paradigm we demonstrate that, for AD HMW and AD SARK tau species, the amplified material is biochemically similar to the original sample. The HMW and SARK materials also show different clearance, propagation kinetics, and propagation patterns. These data indicate the surprising co-occurrence of multiple distinct tau species within the same AD brain, supporting the idea that multiple tau conformers - both fibrillar and nonfibrillar- can impact phenotype in AD.
Keywords: Alzheimer; Kinetics; Seeding; Spreading; Tau.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

