Rotavirus Vaccine Effectiveness Against Severe Acute Gastroenteritis: 2009-2022
- PMID: 39252660
- PMCID: PMC11866101
- DOI: 10.1542/peds.2024-066879
Rotavirus Vaccine Effectiveness Against Severe Acute Gastroenteritis: 2009-2022
Abstract
Background: Rotavirus was the leading cause of acute gastroenteritis among US children until vaccine introduction in 2006, after which, substantial declines in severe rotavirus disease occurred. We evaluated rotavirus vaccine effectiveness (VE) over 13 years (2009-2022).
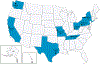
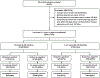
Methods: We analyzed data from the New Vaccine Surveillance Network using a test-negative case-control design to estimate rotavirus VE against laboratory-confirmed rotavirus infections among children seeking care for acute gastroenteritis (≥3 diarrhea or ≥1 vomiting episodes within 24 hours) in the emergency department (ED) or hospital. Case-patients and control-patients were children whose stool specimens tested rotavirus positive or negative, respectively, by enzyme immunoassay or polymerase chain reaction assays. VE was calculated as (1-adjusted odds ratio)×100%. Adjusted odds ratios were calculated by multivariable unconditional logistic regression.
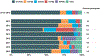
Results: Among 16 188 enrolled children age 8 to 59 months, 1720 (11%) tested positive for rotavirus. Case-patients were less often vaccinated against rotavirus than control-patients (62% versus 88%). VE for receiving ≥1 dose against rotavirus-associated ED visits or hospitalization was 78% (95% confidence interval [CI] 75%-80%). Stratifying by a modified Vesikari Severity Score, VE was 59% (95% CI 49%-67%), 80% (95% CI 77%-83%), and 94% (95% CI 90%-97%) against mild, moderately severe, and very severe disease, respectively. Rotavirus vaccines conferred protection against common circulating genotypes (G1P[8], G2P[4], G3P[8], G9P[8], and G12[P8]). VE was higher in children <3 years (73% to 88%); protection decreased as age increased.
Conclusions: Rotavirus vaccines remain highly effective in preventing ED visits and hospitalizations in US children.
Copyright © 2024 by the American Academy of Pediatrics.
Conflict of interest statement
Dr Weinberg has research support from the New York State Department of Health AIDS Institute and has received honoraria from Merck and Inhalon Biopharma; Dr Englund has research support from AstraZeneca, Merck, Pfizer, and GlaxoSmithKline and consults for AstraZeneca, GlaxoSmithKline, Pfizer, and Sanofi Pasteur; Dr Halasa has research support from Sanofi Pasteur and Quidel and an education grant from Genetech; Dr Harrison received honoraria from WebMD, and his institution received funding for research for in which he was an investigator from GSK, Merck, and Pfizer up to July 1, 2022; Dr Staat has research support from the National Institutes of Health, Merck, Pfizer, and Cepheid and has received honoraria from UpToDate; Dr Schlaudecker has research support from the National Institutes of Health and Pfizer and has received honoraria from Sanofi Pasteur; Dr Zerr received research support from Merck and honoraria from UpToDate and served as a consultant for AlloVir; and the other authors have no conflicts of interest relevant to this article to disclose.
Figures





References
-
- Cortese MM, Parashar UD, Centers for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2009; 58(Rr-2):1–25 - PubMed
-
- Staat MA, Payne DC, Halasa N, et al. Continued evidence of the impact of rotavirus vaccine in children less than 3 years of age from the United States New Vaccine Surveillance Network: a multisite active surveillance program, 2006–2016. Clin Infect Dis. 2020;71(9):e421–e429 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

