Long-term course of ambulatory patients with COVID-19 initially treated with enoxaparin vs no anticoagulation: final analysis of the OVID (enoxaparin for outpatients with COVID-19) randomized trial
- PMID: 39252826
- PMCID: PMC11381852
- DOI: 10.1016/j.rpth.2024.102534
Long-term course of ambulatory patients with COVID-19 initially treated with enoxaparin vs no anticoagulation: final analysis of the OVID (enoxaparin for outpatients with COVID-19) randomized trial
Abstract
Background: Early thromboprophylaxis does not prevent hospital admissions and death among outpatients with symptomatic COVID-19. Its impact on long-term outcomes, including long COVID symptoms and performance status, is unknown.
Objectives: To assess the long-term effects of thromboprophylaxis given at the time of acute COVID-19 in outpatients.
Methods: The OVID (enoxaparin for outpatients with COVID-19) trial randomized outpatients older than 50 years with acute COVID-19 to receive either subcutaneous enoxaparin 40 mg once daily for 14 days or standard of care (no thromboprophylaxis). In this follow-up study, we assessed the 2-year outcomes, including all-cause hospitalization and death, cardiovascular events, long COVID symptoms, and functional limitations based on the Post-COVID-19 Functional Status (PCFS) scale and EuroQol-5 Dimensions-5 Levels scale.
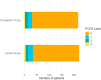
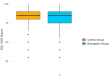
Results: Of 469 potentially eligible patients, 468 survived, of whom 439 (mean age 59 years; 54% men) participated in the Post-OVID study. There was no difference in terms of hospitalization and death (8.3% in the treatment group vs 10% in controls; relative risk, 0.83; 95% CI, 0.5-1.5) and of cardiovascular events between groups. The risk of presenting with long COVID symptoms was similar in the 2 groups (44% in the treatment group vs 47% in the standard of care group), with no difference between groups also concerning individual symptoms. A PCFS grade of 1 to 3, indicating light-to-moderate functional limitation, was recorded in 15% of patients in each group (odds ratio, 0.98; 95% CI, 0.6-1.7). No patients reported severe limitations (PCFS grade 4). Median EuroQol visual analog scale score was 85 on 100 points (IQR, 80-90 for the standard of care group and 75-90 for the enoxaparin group).
Conclusion: Early thromboprophylaxis does not improve long-term, 2-year clinical and functional outcomes among symptomatic ambulatory patients with acute COVID-19.
Keywords: COVID-19; heparin; long COVID; quality of life; thrombosis.
© 2024 The Author(s).
Figures



References
-
- Cares-Marambio K., Montenegro-Jiménez Y., Torres-Castro R., Vera-Uribe R., Torralba Y., Alsina-Restoy X., et al. Prevalence of potential respiratory symptoms in survivors of hospital admission after coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Chron Respir Dis. 2021;18 doi: 10.1177/14799731211002240. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

