Adebrelimab plus chemotherapy and sequential thoracic radiotherapy as first-line therapy for extensive-stage small-cell lung cancer (ES-SCLC): a phase II trial
- PMID: 39252865
- PMCID: PMC11381814
- DOI: 10.1016/j.eclinm.2024.102795
Adebrelimab plus chemotherapy and sequential thoracic radiotherapy as first-line therapy for extensive-stage small-cell lung cancer (ES-SCLC): a phase II trial
Abstract
Background: This phase II prospective trial aimed to investigate the efficacy and safety of adebrelimab (PD-L1 antibody) plus first-line chemotherapy followed by sequential thoracic radiotherapy (TRT) combined with adebrelimab in extensive-stage small-cell lung cancer (ES-SCLC). Biomarkers associated with potential therapeutic effects were also explored.
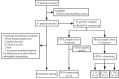
Methods: Patients with previously untreated ES-SCLC were enrolled at Shandong Cancer Hospital and Institute (Jinan, China). Patients received 4-6 cycles of adebrelimab (20 mg/kg, D1, Q3W) combined with EP/EC (etoposide, 100 mg/m2, D1-3, Q3W and cisplatin, 75 mg/m2, D1, Q3W or carboplatin, AUC = 5, D1, Q3W). Then patients with response sequentially underwent consolidative TRT (≥30 Gy in 10 fractions or ≥50 Gy in 25 fractions, involved-field irradiation), and maintenance adebrelimab until disease progression or intolerable adverse events (AEs). The primary endpoint was overall survival (OS). Genomic and circulating tumour DNA (ctDNA) profiling were also analyzed with tumour tissues and peripheral blood. This trial was registered with ClinicalTrials.gov, NCT04562337.
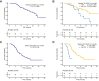
Findings: From October 2020 to April 2023, 67 patients diagnosed with ES-SCLC were enrolled and received at least one dose of study treatment. All patients were included in the efficacy and safety analyses. 45 patients received sequential TRT as planned. The median OS and progression-free survival (PFS) was 21.4 months (95% CI: 17.2-not reached months) and 10.1 months (95% CI: 6.9-15.5 months), respectively. The confirmed objective response rate was 71.6% (48/67, 95% CI: 59.3-82.0%) and disease control rate was 89.6% (60/67, 95% CI: 79.7-95.7%). There were no treatment-related deaths. The most common grade 3 or higher treatment-related adverse events (TRAEs) were hematological toxicities. The incidence of any grade and G3+ pneumonitis was 25% (17/67) and 6% (4/67), respectively. No unexpected adverse events were observed. Patients without co-mutations of TP53/RB1 in both tissue and peripheral blood displayed longer PFS (tissue, P = 0.071; ctDNA, P = 0.060) and OS (tissue, P = 0.032; ctDNA, P = 0.031).
Interpretation: Adebrelimab plus chemotherapy and sequential TRT as first-line therapy for ES-SCLC showed promising efficacy and acceptable safety.
Funding: This study was funded by the National Natural Science Foundation of China (82172865), Jiangsu Hengrui Pharmaceuticals Co., Ltd. and Amoy Diagnostics Co., Ltd.
Keywords: Adebrelimab; ES-SCLC; Immunotherapy; Predictive biomarker; Radiotherapy.
© 2024 The Authors.
Conflict of interest statement
CHZ and JJY are employees of Jiangsu Hengrui Pharmaceuticals. CBZ and QL are employees of Amoy Diagnostics. All other authors declare that they have no competing interests.
Figures


References
-
- Leiter A., Veluswamy R.R., Wisnivesky J.P. The global burden of lung cancer: current status and future trends. Nat Rev Clin Oncol. 2023;20(9):624–639. - PubMed
-
- Stahel R., Thatcher N., Früh M., et al. 1st ESMO Consensus Conference in lung cancer; Lugano 2010: small-cell lung cancer. Ann Oncol. 2011;22(9):1973–1980. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

