A randomized, double-blind phase 2b trial to evaluate efficacy of ChAd63-KH for treatment of post kala-azar dermal leishmaniasis
- PMID: 39253357
- PMCID: PMC11381778
- DOI: 10.1016/j.omtm.2024.101310
A randomized, double-blind phase 2b trial to evaluate efficacy of ChAd63-KH for treatment of post kala-azar dermal leishmaniasis
Abstract
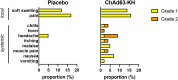
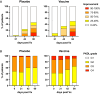
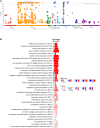
In a recent phase 2a clinical trial, the candidate leishmaniasis vaccine ChAd63-KH was shown to be safe and immunogenic in Sudanese patients with post kala-azar dermal leishmaniasis (PKDL). However, its value as a stand-alone therapeutic was unknown. To assess the therapeutic efficacy of ChAd63-KH, we conducted a randomized, double-blind, placebo-controlled phase 2b trial (ClinicalTrials.gov: NCT03969134). Primary outcomes were safety and efficacy (≥90% improvement in clinical disease). Secondary outcomes were change in severity grade and vaccine-induced immune response. 86 participants with uncomplicated PKDL of ≥6 month duration were randomized to receive ChAd63-KH (7.5 × 1010 viral particles, once by the intramuscular route) or placebo. 75 participants (87%) completed the trial as per protocol. No severe or serious adverse events were observed. At day 90 post-vaccination, 6/40 (15%) and 4/35 (11%) participants in the vaccine and placebo groups, respectively, showed ≥90% clinical improvement (risk ratio [RR] 1.31 [95% confidence interval (CI), 0.40-4.28], p = 0.742). There were also no significant differences in PKDL severity grade between study arms. Whole-blood transcriptomic analysis identified transcriptional modules associated with interferon responses and monocyte and dendritic cell activation. Thus, a single vaccination with ChAd63-KH showed no therapeutic efficacy in this subset of Sudanese patients with PKDL.
Keywords: Leishmania; adenovirus; clinical trial; post kala-azar dermal leishmaniasis; therapeutic; vaccine.
© 2024 The Author(s).
Conflict of interest statement
P.M.K. and C.J.N.L. are co-inventors of a patent that covers the gene insert used in ChAd63-KH.
Figures

References
-
- Zijlstra E.E., el-Hassan A.M. Leishmaniasis in Sudan. Post kala-azar dermal leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2001;95:S59–S76. - PubMed
-
- Zijlstra E.E., Musa A.M., Khalil E.A.G., el-Hassan I.M., el-Hassan A.M. Post-kala-azar dermal leishmaniasis. Lancet Infect. Dis. 2003;3:87–98. - PubMed
-
- Saurabh S., Roy P., Pandey D.K., Ray D., Tarak S., Pandey R., Kumar D., Jamil S., Paulraj A., Kumar A., Dutta S. Changing clinico-epidemiology of post-kala-azar dermal leishmaniasis (PKDL) in India: Results of a survey in four endemic states. J. Vector Borne Dis. 2020;57:161–169. doi: 10.4103/0972-9062.310875. - DOI - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

