This is a preprint.
Sustained fertility from first-wave follicle oocytes that pause their growth
- PMID: 39253445
- PMCID: PMC11383281
- DOI: 10.1101/2024.08.27.609995
Sustained fertility from first-wave follicle oocytes that pause their growth
Abstract
Ovulation results from the cyclical recruitment of non-renewing, quiescent oocytes for growth. Therefore, the primordial follicles that are established during development from an oocyte encapsulated by granulosa cells are thought to comprise the lifelong ovarian reserve 1-4. However, using oocyte lineage tracing in mice, we observed that a subset of oocytes recruited for growth in the first juvenile wave remain paused for many months before continuing growth, ovulation, fertilization and development into healthy offspring. This small subset of genetically-labeled fetal oocytes, labeled with Sycp3-CreERT2, is distinguished by earlier entry and slower dynamics of meiotic prophase I. While labeled oocytes were initially found in both primordial follicles and growing follicles of the first wave, they disappeared from primordial follicles by puberty. Unexpectedly, these first-wave labeled growing oocytes persisted throughout reproductive lifespan and contributed to offspring at a steady rate beyond 12 months of age, suggesting that follicles can pause mid-growth for extended periods then successfully resume. These results challenge the conclusion from lineage tracing of granulosa cells that first-wave follicles make a limited contribution to fertility5 and furthermore suggest that growth-paused oocytes comprise a second and previously unrecognized ovarian reserve.
Figures

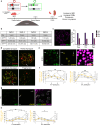
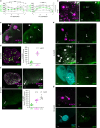


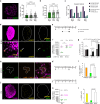
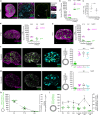
References
-
- Baker T. G. A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond, B, Biol Sci 158, 417–433 (1963). - PubMed
-
- Baker T. G. & Franchi L. L. The fine structure of oogonia and oocytes in human ovaries. J. Cell Sci. 2, 213–224 (1967). - PubMed
-
- Beaumont H. M. & Mandl A. M. A quantitative and cytological study of oogonia and oocytes in the foetal and neonatal rat. Proc. R. Soc. Lond. B. 155, 557–579 (1962).
-
- Pepling M. E. & Spradling A. C. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev. Biol. 234, 339–351 (2001). - PubMed
-
- Zheng W., Zhang H. & Liu K. The two classes of primordial follicles in the mouse ovary: their development, physiological functions and implications for future research. Mol. Hum. Reprod. 20, 286–292 (2014). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
