This is a preprint.
PD-linked LRRK2 G2019S mutation impairs astrocyte morphology and synapse maintenance via ERM hyperphosphorylation
- PMID: 39253496
- PMCID: PMC11383028
- DOI: 10.1101/2023.04.09.536178
PD-linked LRRK2 G2019S mutation impairs astrocyte morphology and synapse maintenance via ERM hyperphosphorylation
Abstract
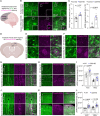
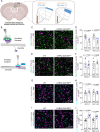
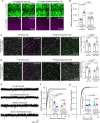
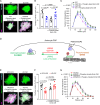
Astrocytes are highly complex cells that mediate critical roles in synapse formation and maintenance by establishing thousands of direct contacts with synapses through their perisynaptic processes. Here, we found that the most common Parkinsonism gene mutation, LRRK2 G2019S, enhances the phosphorylation of the ERM proteins (Ezrin, Radixin, and Moesin), components of the perisynaptic astrocyte processes in a subset of cortical astrocytes. The ERM hyperphosphorylation was accompanied by decreased astrocyte morphological complexity and reduced excitatory synapse density and function. Dampening ERM phosphorylation levels in LRRK2 G2019S mouse astrocytes restored both their morphology and the excitatory synapse density in the anterior cingulate cortex. To determine how LRRK2 mutation impacts Ezrin interactome, we used an in vivo BioID proteomic approach, and we found that astrocytic Ezrin interacts with Atg7, a master regulator of autophagy. The Ezrin/Atg7 interaction is inhibited by Ezrin phosphorylation, thus diminished in LRRK2 G2019S astrocytes. Importantly, the Atg7 function is required to maintain proper astrocyte morphology. Our data provide a molecular pathway through which the LRRK2 G2019S mutation alters astrocyte morphology and synaptic density in a brain-region-specific manner.
Conflict of interest statement
DECLARATION OF INTERESTS The authors declare no competing interests.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
