This is a preprint.
Ultra-Long-Term Delivery of Hydrophilic Drugs Using Injectable In Situ Cross-Linked Depots
- PMID: 39253509
- PMCID: PMC11382995
- DOI: 10.1101/2023.11.04.565631
Ultra-Long-Term Delivery of Hydrophilic Drugs Using Injectable In Situ Cross-Linked Depots
Abstract
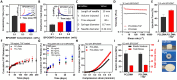
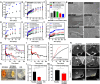
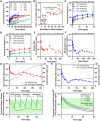
Achieving ultra-long-term release of hydrophilic drugs over several months remains a significant challenge for existing long-acting injectables (LAIs). Existing platforms, such as in situ forming implants (ISFI), exhibit high burst release due to solvent efflux and microsphere-based approaches lead to rapid drug diffusion due to significant water exchange and large pores. Addressing these challenges, we have developed an injectable platform that, for the first time, achieves ultra-long-term release of hydrophilic drugs for over six months. This system employs a methacrylated ultra-low molecular weight pre-polymer (polycaprolactone) to create in situ cross-linked depots (ISCD). The ISCD's solvent-free design and dense mesh network, both attributed to the ultra-low molecular weight of the pre-polymer, effectively minimizes burst release and water influx/efflux. In vivo studies in rats demonstrate that ISCD outperforms ISFI by achieving lower burst release and prolonged drug release. We demonstrated the versatility of ISCD by showcasing ultra-long-term delivery of several hydrophilic drugs, including antiretrovirals (tenofovir alafenamide, emtricitabine, abacavir, and lamivudine), antibiotics (vancomycin and amoxicillin) and an opioid antagonist naltrexone. Additionally, ISCD achieved ultra-long-term release of the hydrophobic drug tacrolimus and enabled co-delivery of hydrophilic drug combinations encapsulated in a single depot. We also identified design parameters to tailor the polymer network, tuning drug release kinetics and ISCD degradation. Pharmacokinetic modeling predicted over six months of drug release in humans, significantly surpassing the one-month standard achievable for hydrophilic drugs with existing LAIs. The platform's biodegradability, retrievability, and biocompatibility further underscore its potential for improving treatment adherence in chronic conditions.
Conflict of interest statement
Competing interests S.L., S.Z, J.M.K. and N.J. have one pending patent based on the ISCD formulation described in this manuscript. J.M.K has been a paid consultant and or equity holder for companies (listed here: https://www.karplab.net/team/jeff-karp) including biotechnologies companies such as Stempeutics, Sanofi, Celltex, LifeVaultBio, Takeda, Ligandal, Camden Partners, Stemgent, Biogen, Pancryos, Element Biosciences, Frequency Therapeutics, Corner Therapeutics, Quthero, and Mesoblast. J.M.K. has been a paid consultant and or equity holder for multiple biotechnology companies. The interests of J.M.K. were reviewed and are subject to a management plan overseen by his institution in accordance with its conflict of interest policies.
Figures


References
-
- McLellan A.T., Lewis D.C., O’brien C.P. & Kleber H.D. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. Jama 284, 1689–1695 (2000). - PubMed
-
- Sabaté E. Adherence to long-term therapies: evidence for action. (World Health Organization, 2003).
-
- Osterberg L. & Blaschke T. Adherence to medication. New England journal of medicine 353, 487–497 (2005). - PubMed
-
- Swindells S. et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. New England Journal of Medicine 382, 1112–1123 (2020). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
