Impact of swine influenza A virus on porcine reproductive and respiratory syndrome virus infection in alveolar macrophages
- PMID: 39253525
- PMCID: PMC11381391
- DOI: 10.3389/fvets.2024.1454762
Impact of swine influenza A virus on porcine reproductive and respiratory syndrome virus infection in alveolar macrophages
Abstract
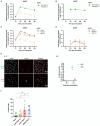
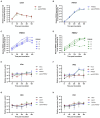
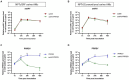
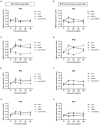
Porcine respiratory disease complex represents a major challenge for the swine industry, with swine influenza A virus (swIAV) and porcine reproductive and respiratory syndrome virus (PRRSV) being major contributors. Epidemiological studies have confirmed the co-circulation of these viruses in pig herds, making swIAV-PRRSV co-infections expected. A couple of in vivo co-infection studies have reported replication interferences between these two viruses. Herein, using a reductionist in vitro model, we investigated the potential mechanisms of these in vivo interferences. We first examined the impact of swIAV on porcine alveolar macrophages (AMs) and its effects on AMs co-infection by PRRSV. This was done either in monoculture or in co-culture with respiratory tracheal epithelial cells to represent the complexity of the interactions between the viruses and their respective target cells (epithelial cells for swIAV and AMs for PRRSV). AMs were obtained either from conventional or specific pathogen-free (SPF) pigs. SwIAV replication was abortive in AMs, inducing cell death at high multiplicity of infections. In AMs from three out of four conventional animals, swIAV showed no impact on PRRSV replication. However, inhibition of PRRSV multiplication was observed in AMs from one animal, accompanied by an early increase in the expression of interferon (IFN)-I and IFN-stimulated genes. In AMs from six SPF pigs, swIAV inhibited PRRSV replication in all animals, with an early induction of antiviral genes. Co-culture experiments involving tracheal epithelial cells and AMs from either SPF or conventional pigs all showed swIAV-induced inhibition of PRRSV replication, together with early induction of antiviral genes. These findings highlight the complex interactions between swIAV and PRRSV in porcine AMs, and would suggest a role of host factors, such as sanitary status, in modulating viral propagation. Our co-culture experiments demonstrated that swIAV inhibits PRRSV replication more effectively in the presence of respiratory tracheal epithelial cells, suggesting a synergistic antiviral response between AMs and epithelial cells, consistent with in vivo experiments.
Keywords: co-infection; co-inoculation; interferon; lung; porcine respiratory disease complex.
Copyright © 2024 Grevelinger, Bourry, Meurens, Perrin, Hervet, Dubreil, Simon and Bertho.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
LinkOut - more resources
Full Text Sources

