MED12 and CDK8/19 Modulate Androgen Receptor Activity and Enzalutamide Response in Prostate Cancer
- PMID: 39253786
- PMCID: PMC11398899
- DOI: 10.1210/endocr/bqae114
MED12 and CDK8/19 Modulate Androgen Receptor Activity and Enzalutamide Response in Prostate Cancer
Abstract
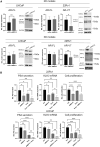
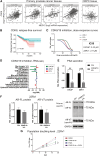
Prostate cancer progression is driven by androgen receptor (AR) activity, which is a target for therapeutic approaches. Enzalutamide is an AR inhibitor that prolongs the survival of patients with advanced prostate cancer. However, resistance mechanisms arise and impair its efficacy. One of these mechanisms is the expression of AR-V7, a constitutively active AR splice variant. The Mediator complex is a multisubunit protein that modulates gene expression on a genome-wide scale. MED12 and cyclin-dependent kinase (CDK)8, or its paralog CDK19, are components of the kinase module that regulates the proliferation of prostate cancer cells. In this study, we investigated how MED12 and CDK8/19 influence cancer-driven processes in prostate cancer cell lines, focusing on AR activity and the enzalutamide response. We inhibited MED12 expression and CDK8/19 activity in LNCaP (AR+, enzalutamide-sensitive), 22Rv1 (AR-V7+, enzalutamide-resistant), and PC3 (AR-, enzalutamide-insensitive) cells. Both MED12 and CDK8/19 inhibition reduced cell proliferation in all cell lines, and MED12 inhibition reduced proliferation in the respective 3D spheroids. MED12 knockdown significantly inhibited c-Myc protein expression and signaling pathways. In 22Rv1 cells, it consistently inhibited the AR response, prostate-specific antigen (PSA) secretion, AR target genes, and AR-V7 expression. Combined with enzalutamide, MED12 inhibition additively decreased the AR activity in both LNCaP and 22Rv1 cells. CDK8/19 inhibition significantly decreased PSA secretion in LNCaP and 22Rv1 cells and, when combined with enzalutamide, additively reduced proliferation in 22Rv1 cells. Our study revealed that MED12 and CDK8/19 regulate AR activity and that their inhibition may modulate response to enzalutamide in prostate cancer.
Keywords: Mediator complex; androgen receptor; enzalutamide; prostate cancer.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society.
Figures



References
-
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187‐1197. - PubMed
-
- Wang Y, Chen J, Wu Z, et al. Mechanisms of enzalutamide resistance in castration-resistant prostate cancer and therapeutic strategies to overcome it. Br J Pharmacol. 2021;178(2):239‐261. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

