Microtubules, Membranes, and Movement: New Roles for Stathmin-2 in Axon Integrity
- PMID: 39253877
- PMCID: PMC11407747
- DOI: 10.1002/jnr.25382
Microtubules, Membranes, and Movement: New Roles for Stathmin-2 in Axon Integrity
Abstract
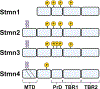
Neurons establish functional connections responsible for how we perceive and react to the world around us. Communication from a neuron to its target cell occurs through a long projection called an axon. Axon distances can exceed 1 m in length in humans and require a dynamic microtubule cytoskeleton for growth during development and maintenance in adulthood. Stathmins are microtubule-associated proteins that function as relays between kinase signaling and microtubule polymerization. In this review, we describe the prolific role of Stathmins in microtubule homeostasis with an emphasis on emerging roles for Stathmin-2 (Stmn2) in axon integrity and neurodegeneration. Stmn2 levels are altered in Amyotrophic Lateral Sclerosis and loss of Stmn2 provokes motor and sensory neuropathies. There is growing potential for employing Stmn2 as a disease biomarker or even a therapeutic target. Meeting this potential requires a mechanistic understanding of emerging complexity in Stmn2 function. In particular, Stmn2 palmitoylation has a surprising contribution to axon maintenance through undefined mechanisms linking membrane association, tubulin interaction, and axon transport. Exploring these connections will reveal new insight on neuronal cell biology and novel opportunities for disease intervention.
Keywords: ALS; Stmn2; axon degeneration; cytoskeleton; neurodegeneration; palmitoylation.
© 2024 The Author(s). Journal of Neuroscience Research published by Wiley Periodicals LLC.
Conflict of interest statement
Conflict of Interest Statement
The authors do not have any conflicts of interests related to the contents of this manuscript to declare.
Figures


References
-
- Anderson D, & Axel R (1985). Molecular Probes for the Development and Plasticity of Neural Crest Derivatives. Cell, 42, 649–662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

