From BBB to PPP: Bioenergetic requirements and challenges for oligodendrocytes in health and disease
- PMID: 39253904
- PMCID: PMC11657931
- DOI: 10.1111/jnc.16219
From BBB to PPP: Bioenergetic requirements and challenges for oligodendrocytes in health and disease
Abstract
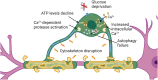
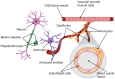
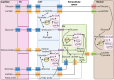
Mature myelinating oligodendrocytes, the cells that produce the myelin sheath that insulates axons in the central nervous system, have distinct energetic and metabolic requirements compared to neurons. Neurons require substantial energy to execute action potentials, while the energy needs of oligodendrocytes are directed toward building the lipid-rich components of myelin and supporting neuronal metabolism by transferring glycolytic products to axons as additional fuel. The utilization of energy metabolites in the brain parenchyma is tightly regulated to meet the needs of different cell types. Disruption of the supply of metabolites can lead to stress and oligodendrocyte injury, contributing to various neurological disorders, including some demyelinating diseases. Understanding the physiological properties, structures, and mechanisms involved in oligodendrocyte energy metabolism, as well as the relationship between oligodendrocytes and neighboring cells, is crucial to investigate the underlying pathophysiology caused by metabolic impairment in these disorders. In this review, we describe the particular physiological properties of oligodendrocyte energy metabolism and the response of oligodendrocytes to metabolic stress. We delineate the relationship between oligodendrocytes and other cells in the context of the neurovascular unit, and the regulation of metabolite supply according to energetic needs. We focus on the specific bioenergetic requirements of oligodendrocytes and address the disruption of metabolic energy in demyelinating diseases. We encourage further studies to increase understanding of the significance of metabolic stress on oligodendrocyte injury, to support the development of novel therapeutic approaches for the treatment of demyelinating diseases.
Keywords: hypoperfusion; metabolic stress; metabolism; multiple sclerosis; myelin; oligodendrocyte injury.
© 2024 The Author(s). Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Figures


References
-
- Abbott, N. J. , Rönnbäck, L. , & Hansson, E. (2006). Astrocyte‐endothelial interactions at the blood‐brain barrier. Nature Reviews. Neuroscience, 7, 41–53. - PubMed
-
- Aboul‐Enein, F. , Rauschka, H. , Kornek, B. , Stadelmann, C. , Stefferl, A. , Brück, W. , Lucchinetti, C. , Schmidbauer, M. , Jellinger, K. , & Lassmann, H. (2003). Preferential loss of myelin‐associated glycoprotein reflects hypoxia‐like white matter damage in stroke and inflammatory brain diseases. Journal of Neuropathology and Experimental Neurology, 62, 25–33. - PubMed
-
- Alarcon‐Martinez, L. , Villafranca‐Baughman, D. , Quintero, H. , Kacerovsky, J. B. , Dotigny, F. , Murai, K. K. , Prat, A. , Drapeau, P. , & Di Polo, A. (2020). Interpericyte tunnelling nanotubes regulate neurovascular coupling. Nature, 585, 91–95. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

