Paternal hypercholesterolemia elicits sex-specific exacerbation of atherosclerosis in offspring
- PMID: 39253968
- PMCID: PMC11385100
- DOI: 10.1172/jci.insight.179291
Paternal hypercholesterolemia elicits sex-specific exacerbation of atherosclerosis in offspring
Abstract
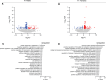
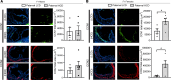
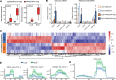
Emerging studies suggest that various parental exposures affect offspring cardiovascular health, yet the specific mechanisms, particularly the influence of paternal cardiovascular disease (CVD) risk factors on offspring cardiovascular health, remain elusive. The present study explores how paternal hypercholesterolemia affects offspring atherosclerosis development using the LDL receptor-deficient (LDLR-/-) mouse model. We found that paternal high-cholesterol diet feeding led to significantly increased atherosclerosis in F1 female, but not male, LDLR-/- offspring. Transcriptomic analysis highlighted that paternal hypercholesterolemia stimulated proatherogenic genes, including Ccn1 and Ccn2, in the intima of female offspring. Sperm small noncoding RNAs (sncRNAs), particularly transfer RNA-derived (tRNA-derived) small RNAs (tsRNAs) and rRNA-derived small RNAs (rsRNAs), contribute to the intergenerational transmission of paternally acquired metabolic phenotypes. Using a newly developed PANDORA-Seq method, we identified that high-cholesterol feeding elicited changes in sperm tsRNA/rsRNA profiles that were undetectable by traditional RNA-Seq, and these altered sperm sncRNAs were potentially key factors mediating paternal hypercholesterolemia-elicited atherogenesis in offspring. Interestingly, high-cholesterol feeding altered sncRNA biogenesis-related gene expression in the epididymis but not testis of LDLR-/- sires; this may have led to the modified sperm sncRNA landscape. Our results underscore the sex-specific intergenerational effect of paternal hypercholesterolemia on offspring cardiovascular health and contribute to the understanding of chronic disease etiology originating from parental exposures.
Keywords: Atherosclerosis; Cardiovascular disease; Cell biology; Epigenetics; Vascular biology.
Conflict of interest statement
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

