Immunomodulators and immunosuppressants for progressive multiple sclerosis: a network meta-analysis
- PMID: 39254048
- PMCID: PMC11384553
- DOI: 10.1002/14651858.CD015443.pub2
Immunomodulators and immunosuppressants for progressive multiple sclerosis: a network meta-analysis
Abstract
Background: In recent years a broader range of immunomodulatory and immunosuppressive treatment options have emerged for people with progressive forms of multiple sclerosis (PMS). While consensus supports these options as reducing relapses, their relative benefit and safety profiles remain unclear due to a lack of direct comparison trials.
Objectives: To compare through network meta-analysis the efficacy and safety of alemtuzumab, azathioprine, cladribine, cyclophosphamide, daclizumab, dimethylfumarate, diroximel fumarate, fingolimod, fludarabine, glatiramer acetate, immunoglobulins, interferon beta 1-a and beta 1-b, interferon beta-1b (Betaferon), interferon beta-1a (Avonex, Rebif), laquinimod, leflunomide, methotrexate, minocycline, mitoxantrone, mycophenolate mofetil, natalizumab, ocrelizumab, ofatumumab, ozanimod, pegylated interferon beta-1a, ponesimod, rituximab, siponimod, corticosteroids, and teriflunomide for PMS.
Search methods: We searched CENTRAL, MEDLINE, and Embase up to August 2022, as well as ClinicalTrials.gov and the WHO ICTRP.
Selection criteria: Randomised controlled trials (RCTs) that studied one or more treatments as monotherapy, compared to placebo or to another active agent, for use in adults with PMS.
Data collection and analysis: Two review authors independently selected studies and extracted data. We performed data synthesis by pair-wise and network meta-analysis. We assessed the certainty of the body of evidence according to GRADE.
Main results: We included 23 studies involving a total of 10,167 participants. The most frequent (39% of studies) reason for a rating of high risk of bias was sponsor role in study authorship and data management and analysis. Other concerns were performance, attrition, and selective reporting bias, with 8.7% of studies at high risk of bias for all three of these domains. The common comparator for network analysis was placebo. Relapses over 12 months: assessed in one study (318 participants). None of the treatments assessed showed moderate or high certainty evidence compared to placebo. Relapses over 24 months: assessed in six studies (1622 participants). The number of people with clinical relapses is probably trivially reduced with rituximab (risk ratio (RR) 0.60, 95% confidence interval (CI) 0.19 to 1.95; moderate certainty evidence). None of the remaining treatments assessed showed moderate or high certainty evidence compared to placebo. Relapses over 36 months: assessed in four studies (2095 participants). The number of people with clinical relapses is probably trivially reduced with interferon beta-1b (RR 0.82, 95% CI 0.73 to 0.93; moderate certainty evidence). None of the remaining treatments assessed showed moderate or high certainty evidence compared to placebo. Disability worsening over 24 months: assessed in 11 studies (5284 participants). None of the treatments assessed showed moderate or high certainty evidence compared to placebo. Disability worsening over 36 months: assessed in five studies (2827 participants). None of the treatments assessed showed moderate or high certainty evidence compared to placebo. Serious adverse events: assessed in 15 studies (8019 participants). None of the treatments assessed showed moderate or high certainty evidence compared to placebo. Discontinuation due to adverse events: assessed in 21 studies (9981 participants). The number of people who discontinued treatment due to adverse events is trivially increased with interferon beta-1a (odds ratio (OR) 2.93, 95% CI 1.64 to 5.26; high certainty evidence). The number of people who discontinued treatment due to adverse events is probably trivially increased with rituximab (OR 4.00, 95% CI 0.84 to 19.12; moderate certainty evidence); interferon beta-1b (OR 2.98, 95% CI 1.92 to 4.61; moderate certainty evidence); immunoglobulins (OR 1.95, 95% CI 0.99 to 3.84; moderate certainty evidence); glatiramer acetate (OR 3.98, 95% CI 1.48 to 10.72; moderate certainty evidence); natalizumab (OR 1.02, 95% CI 0.55 to 1.90; moderate certainty evidence); siponimod (OR 1.53, 95% CI 0.98 to 2.38; moderate certainty evidence); fingolimod (OR 2.29, 95% CI 1.46 to 3.60; moderate certainty evidence), and ocrelizumab (OR 1.24, 95% CI 0.54 to 2.86; moderate certainty evidence). None of the remaining treatments assessed showed moderate or high certainty evidence compared to placebo.
Authors' conclusions: The number of people with PMS with relapses is probably slightly reduced with rituximab at two years, and interferon beta-1b at three years, compared to placebo. Both drugs are also probably associated with a slightly higher proportion of withdrawals due to adverse events, as are immunoglobulins, glatiramer acetate, natalizumab, fingolimod, siponimod, and ocrelizumab; we have high confidence that this is the case with interferon beta-1a. We found only low or very low certainty evidence relating to disability progression for the included disease-modifying treatments compared to placebo, largely due to imprecision. We are also uncertain about the effect of interventions on serious adverse events, also because of imprecision. These findings are due in part to the short follow-up of the included RCTs, which lacked detection of less common severe adverse events. Moreover, the funding source of many included studies may have introduced bias into the results. Future research on PMS should include head-to-head rather than placebo-controlled trials, with a longer follow-up of at least three years. Given the relative rarity of PMS, controlled, non-randomised studies on large samples may usefully integrate data from pivotal RCTs. Outcomes valuable and meaningful to people with PMS should be consistently adopted and measured to permit the evaluation of relative effectiveness among treatments.
Copyright © 2024 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
BR: has worked as Managing Editor for the Cochrane Multiple Sclerosis and Rare Disease of the CNS Review Group and Cochrane Central Editorial Service. He was not involved in the editorial process of the current review.
SM: is the Joint‐Coordinating Editor of the Cochrane Drugs and Alcohol Group. She was not involved in the editorial process of the current review. She received funding from the Multiple Sclerosis International Federation to perform data extraction, risk of bias assessment, and assessment of the certainty of evidence.
MGL: received funding from Multiple Sclerosis International Federation to perform data extraction, risk of bias assessment, and assessment of the certainty of evidence.
CDG: received funding from the Multiple Sclerosis International Federation to perform the statistical analyses.
TP: received funding from the Multiple Sclerosis International Federation to contribute to the review.
GF: is the Joint‐Coordinating Editor of Cochrane Multiple Sclerosis and Rare Disease of the CNS Review Group. She was not involved in the editorial process of the current review. GF has been directly involved in a study that may meet the inclusion criteria for the review. In line with Cochrane's conflict of interest policy, GF was not involved in defining the overall inclusion and exclusion criteria for the review or in actions relating to their study when conducting the full review.
GP: has published opinions on the methodology of conducting interventions in the context of multiple sclerosis; has worked as an independent contractor with the Multiple Sclerosis Society, Bristol‐Myers Squibb, and Multiple Sclerosis International Federation; and has been involved in Data and Safety Monitoring with the UK National Institute for Health and Care Research.
MF: works as consultant neurologist in an inpatient clinic; has published opinions in a medical journal on other pharmaceuticals; and has received travel and meeting attendance support form Novartis, Merck, Biogen, Roche, and Sanofi Genzyme.
IT: has been directly involved in a study that may meet the inclusion criteria for the review. In line with Cochrane's conflict of interest policy, IT was not involved in defining the overall inclusion and exclusion criteria for the review or in actions relating to their study when conducting the full review.
EB: has worked as a health professional in an outpatient clinic; has published opinions in a medical journal on other pharmaceuticals; and has received travel and meeting attendance support from Roche, Sanofi Genzyme, and Biogen.
FN: is the Coordinating Editor of the Cochrane Multiple Sclerosis and Rare Disease of the CNS Review Group. He was not involved in the editorial process of the current review.
Figures
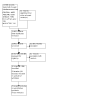

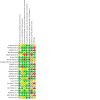

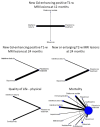







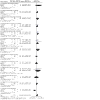









Update of
- doi: 10.1002/14651858.CD015443
References
References to studies included in this review
Anderson 2004 {published data only}
-
- Andersen O, Elovaara I, Färkkilä M, Hansen HJ, Mellgren SI, Myhr KM, Sandberg-Wollheim M, Soelberg Sørensen P. Multicentre, randomised, double blind, placebo controlled, phase III study of weekly, low dose, subcutaneous interferon beta-1a in secondary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry 2004 ;75(5):706-10. [DOI: 10.1136/jnnp.2003.010090] [PMID: ] - DOI - PMC - PubMed
ARPEGGIO 2020 {published data only}
-
- A Phase 2 Clinical Study in Subjects With Primary Progressive Multiple Sclerosis to Assess the Efficacy, Safety and Tolerability of Two Oral Doses of Laquinimod Either of 0.6 mg/Day or 1.5mg/Day (Experimental Drug) as Compared to Placebo (ARPEGGIO). https://clinicaltrials.gov/ct2/show/NCT02284568.
-
- Giovannoni G, Knappertz V, Steinerman JR, Tansy AP, Li T, Krieger S, Uccelli A, Uitdehaag BMJ, Montalban X, Hartung HP, Pia Sormani M, Cree BAC, Lublin F, Barkhof F. A randomized, placebo-controlled, phase 2 trial of laquinimod in primary progressive multiple sclerosis. Neurology 2020;95(8):e1027-e1040. [DOI: 10.1212/WNL.0000000000010284] [PMID: ] - DOI - PubMed
ASCEND 2018 {published data only}
-
- Kapoor R, Ho PR, Campbell N, Chang I, Deykin A, Forrestal F, Lucas N, Yu B, Arnold DL, Freedman MS, Goldman MD, Hartung HP, Havrdová EK, Jeffery D, Miller A, Sellebjerg F, Cadavid D, Mikol D, Steiner D, ASCEND investigators. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol 2018;17(5):405-415. [DOI: 10.1016/S1474-4422(18)30069-3] [PMID: ] - DOI - PubMed
Bornstein 1991 {published data only}
-
- Bornstein MB, Miller A, Slagle S, Weitzman M, Drexler E, Keilson M, Spada V, Weiss W, Appel S, Rolak L, et al. A placebo-controlled, double-blind, randomized, two-center, pilot trial of Cop 1 in chronic progressive multiple sclerosis. Neurology 1991;41(4):533-9. [DOI: 10.1212/wnl.41.4.533] [PMID: ] - DOI - PubMed
Cheshmavar 2020 {published data only}
Ellison 1989 {published data only}
-
- Ellison GW, Myers LW, Mickey MR, Graves MC, Tourtellotte WW, Syndulko K, Holevoet-Howson MI, Lerner CD, Frane MV, Pettler-Jennings P. A placebo-controlled, randomized, double-masked, variable dosage, clinical trial of azathioprine with and without methylprednisolone in multiple sclerosis. Neurology 1989;39(8):1018-26. [DOI: 10.1212/wnl.39.8.1018] [PMID: ] - DOI - PubMed
Etemadifar 2019 {published data only}
-
- Etemadifar M, Ghourchian S, Mahinparvar N, Salari M, Etemadifar F, Nikanpour Y, Sanaei S, Akbari M. Cyclophosphamide Versus Rituximab in Progressive Forms of Multiple Sclerosis. Acta Med Iran 2020;57(8):484-491.
European Study Group 1998 {published data only}
-
- European Study Group on interferon beta-1b in secondary progressive MS. Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. Lancet 1998;352(9139):1491-7. [PMID: ] - PubMed
EXPAND 2018 {published data only}
-
- Benedict RHB, Tomic D, Cree BA, Fox R, Giovannoni G, Bar-Or A, Gold R, Vermersch P, Pohlmann H, Wright I, Karlsson G, Dahlke F, Wolf C, Kappos L. Siponimod and Cognition in Secondary Progressive Multiple Sclerosis: EXPAND Secondary Analyses. Neurology 2021;96(3):e376-e386. [DOI: 10.1212/WNL.0000000000011275] [PMID: ] - DOI - PubMed
-
- Kappos L, Bar-Or A, Cree BAC, Fox RJ, Giovannoni G, Gold R, Vermersch P, Arnold DL, Arnould S, Scherz T, Wolf C, Wallström E, Dahlke F, EXPAND Clinical Investigators. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet 2018;391(10127):1263-1273. [DOI: 10.1016/S0140-6736(18)30475-6] - DOI - PubMed
Goodkin 1995 {published data only}
Hawker 2009 {published data only}
-
- Hawker K, O'Connor P, Freedman MS, Calabresi PA, Antel J, Simon J, Hauser S, Waubant E, Vollmer T, Panitch H, Zhang J, Chin P, Smith CH, OLYMPUS trial group. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol 2009;66(4):460-71. [DOI: 10.1002/ana.21867] [PMID: ] - DOI - PubMed
Hommes 2004 {published data only}
IMPACT 2002 {published data only}
-
- Cohen JA, Cutter GR, Fischer JS, Goodman AD, Heidenreich FR, Kooijmans MF, Sandrock AW, Rudick RA, Simon JH, Simonian NA, Tsao EC, Whitaker JN, IMPACT Investigators. Benefit of interferon beta-1a on MSFC progression in secondary progressive MS. Neurology 2002;59(5):679-87. [DOI: 10.1212/wnl.59.5.679] [PMID: ] - DOI - PubMed
INFORMS 2016 {published data only}
-
- Fox EJ, Lublin FD, Wolinsky JS, Cohen JA, Williams IM, Meng X, Ziehn M, Kolodny S, Cree BAC. Lymphocyte counts and infection rates: Long-term fingolimod treatment in primary progressive MS. Neurol Neuroimmunol Neuroinflamm 2019;6(6):e614. [DOI: 10.1212/NXI.0000000000000614] [PMID: ] - DOI - PMC - PubMed
-
- Lublin F, Miller DH, Freedman MS, Cree BAC, Wolinsky JS, Weiner H, Lubetzki C, Hartung HP, Montalban X, Uitdehaag BMJ, Merschhemke M, Li B, Putzki N, Liu FC, Häring DA, Kappos L, INFORMS study investigators. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet 2016;387(10023):1075-1084. [DOI: 10.1016/S0140-6736(15)01314-8] - DOI - PubMed
Komori 2016 {published data only}
Leary 2003 {published data only}
Montalban 2009 {published data only}
-
- Montalban X, Sastre-Garriga J, Tintoré M, Brieva L, Aymerich FX, Río J, Porcel J, Borràs C, Nos C, Rovira A. A single-center, randomized, double-blind, placebo-controlled study of interferon beta-1b on primary progressive and transitional multiple sclerosis. Mult Scler 2009;15(10):1195-205. [DOI: 10.1177/1352458509106937] [PMID: ] - DOI - PubMed
NASP 2004 {published data only}
-
- Panitch H, Miller A, Paty D, Weinshenker B, North American Study Group on Interferon beta-1b in Secondary Progressive MS. Interferon beta-1b in secondary progressive MS: results from a 3-year controlled study. Neurology 2004;63(10):1788-95. [DOI: 10.1212/01.wnl.0000146958.77317.3e.] [PMID: ] - DOI - PubMed
ORATORIO 2017 {published data only}
-
- Fox EJ, Markowitz C, Applebee A, Montalban X, Wolinsky JS, Belachew S, Fiore D, Pei J, Musch B, Giovannoni G. Ocrelizumab reduces progression of upper extremity impairment in patients with primary progressive multiple sclerosis: Findings from the phase III randomized ORATORIO trial. Mult Scler 2018;24(14):1862-1870. [DOI: 10.1177/1352458518808189] [PMID: ] - DOI - PMC - PubMed
-
- Mayer L, Kappos L, Racke MK, Rammohan K, Traboulsee A, Hauser SL, Julian L, Köndgen H, Li C, Napieralski J, Zheng H, Wolinsky JS. Ocrelizumab infusion experience in patients with relapsing and primary progressive multiple sclerosis: Results from the phase 3 randomized OPERA I, OPERA II, and ORATORIO studies. Mult Scler Relat Disord 2019;30:236-243. [DOI: 10.1016/j.msard.2019.01.044] [PMID: ] - DOI - PubMed
-
- Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, Seze J, Giovannoni G, Hartung HP, Hemmer B, Lublin F, Rammohan KW, Selmaj K, Traboulsee A, Sauter A, Masterman D, Fontoura P, Belachew S, Garren H, Mairon N, Chin P, Wolinsky JS, ORATORIO Clinical Investigators. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N Engl J Med 2017;376(3):209-220. [DOI: 10.1056/NEJMoa1606468] [PMID: ] - DOI - PubMed
-
- Wolinsky JS, Montalban X, Hauser SL, Giovannoni G, Vermersch P, Bernasconi C, Deol-Bhullar G, Garren H, Chin P, Belachew S, Kappos L. Evaluation of no evidence of progression or active disease (NEPAD) in patients with primary progressive multiple sclerosis in the ORATORIO trial. Ann Neurol 2018;84(4):527-536. [DOI: 10.1002/ana.25313] [PMID: ] - DOI - PMC - PubMed
Pohlau 2007 {published data only}
-
- Pöhlau D, Przuntek H, Sailer M, Bethke F, Koehler J, König N, Heesen C, Späth P, Andresen I. Intravenous immunoglobulin in primary and secondary chronic progressive multiple sclerosis: a randomized placebo controlled multicentre study. Mult Scler 2007;13(9):1107-17. [DOI: 10.1177/1352458507078400] [PMID: ] - DOI - PubMed
PROMESS 2017 {published data only}
-
- Brochet B, Deloire MS, Perez P, Loock T, Baschet L, Debouverie M, Pittion S, Ouallet JC, Clavelou P, Sèze J, Collongues N, Vermersch P, Zéphir H, Castelnovo G, Labauge P, Lebrun C, Cohen M, Ruet A, PROMESS study investigators. Double-Blind Controlled Randomized Trial of Cyclophosphamide versus Methylprednisolone in Secondary Progressive Multiple Sclerosis. PLoS One 2017;12(1):e0168834. [DOI: 10.1371/journal.pone.0168834] [PMID: ] - DOI - PMC - PubMed
SPECTRIMS 2001 {published data only}
Wolinsky 2007 {published data only}
-
- Wolinsky JS, Narayana PA, O'Connor P, Coyle PK, Ford C, Johnson K, Miller A, Pardo L, Kadosh S, Ladkani D, PROMiSe Trial Study Group. Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann Neurol 2007;61(1):14-24. [DOI: 10.1002/ana.21079] [PMID: ] - DOI - PubMed
References to studies excluded from this review
British and Dutch 1988 {published data only}
-
- The British, Dutch MSATG. Double-masked trial of azathioprine in multiple sclerosis. Lancet 1988;2(8604):179–83. - PubMed
CCMSSG 1991 {published data only}
-
- CCMSSG. The Canadian cooperative trial of cyclophosphamide and plasma exchange in progressive multiple sclerosis. Lancet 1991;337(8739):441-6. - PubMed
Edan 1997 {published data only}
-
- Edan G, Miller D, Clanet M, Confavreux C, Lyon-Caen O, Lubetzki C, et al. Therapeutic effect of mitoxantrone combined with methylprednisolone in multiple sclerosis: a randomised multicentre study of active disease using MRI and clinical criteria. Journal of Neurology, Neurosurgery, and Psychiatry 1997;62(2):112–8. - PMC - PubMed
Evdoshenko 2019 {published data only}
-
- Evdoshenko EP, Neofidov NA, Bakhtiyarova KZ, Davydovskaya MV, Kairbekova EI, Kolontareva YM, Malkova NA, Odinak MM, Popova EV, Sazonov DV, Stolyarov ID, Smagina IV, Fedyanin AS, Habirov FA, Khaibullin TI, Khachanova NV, Shchukin IA, Boyko AN. The efficacy and safety of siponimod in the Russian population of patients with secondary progressive multiple sclerosis [Éffektivnost' i bezopasnost' siponimoda u patsientov s vtorichno-progressiruiushchim rasseiannym sklerozom v rossiĭskoĭ populiatsii]. Zh Nevrol Psikhiatr Im S S Korsakova 2019;119(10. Vyp. 2):110-119. [DOI: 10.17116/jnevro201911910110] [PMID: ] - DOI - PubMed
Fox 2018 {published data only}
-
- Fox EJ, Markowitz C, Applebee A, Montalban X, Wolinsky JS, Belachew S, Fiore D, Pei J, Musch B, Giovannoni G. Ocrelizumab reduces progression of upper extremity impairment in patients with primary progressive multiple sclerosis: Findings from the phase III randomized ORATORIO trial. Mult Scler 2018;24(14):1862-1870. [DOI: 10.1177/1352458518808189] [PMID: ] - DOI - PMC - PubMed
Ghezzi 1989 {published data only}
-
- Ghezzi A, Di Falco M, Locatelli C. Clinical controlled randomized trial of azathioprine in multiple sclerosis. In: Gonsette RE, Delmotte P, editors(s). Recent Advances in Multiple Sclerosis Therapy. Amsterdam: Elsevier, 1989.
Hartung 2002 {published data only}
-
- Hartung H, Gonsette R, Konig N, Kwiecinski H, Guseo A, Morrissey S, et al. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet 2002;360(9350):2018-25. [PMID: ] - PubMed
Kuhle 2016 {published data only}
-
- Kuhle J, Hardmeier M, Disanto G, Gugleta K, Ecsedi M, Lienert C, Amato MP, Baum K, Buttmann M, Bayas A, Brassat D, Brochet B, Confavreux C, Edan G, Färkkilä M, Fredrikson S, Frontoni M, D'Hooghe M, Hutchinson M, De Keyser J, Kieseier BC, Kümpfel T, Rio J, Polman C, Roullet E, Stolz C, Vass K, Wandinger KP, Kappos L, European Long-term Follow-up Study Group in Interferon β-1b in Secondary-progressive Multiple Sclerosis. A 10-year follow-up of the European multicenter trial of interferon β-1b in secondary-progressive multiple sclerosis. Mult Scler 2016 ;22(4):533-43. [DOI: 10.1177/1352458515594440] [PMID: ] - DOI - PubMed
Milanese 1993 {published data only}
-
- Milanese C, La Mantia L, Salmaggi A, Eoli M. A double blind study on azathioprine efficacy in multiple sclerosis: final report. Journal of Neurology 1993;240(5):295-8. - PubMed
Miller 1961 {published data only}
-
- Miller H, Newell D, Ridley A. Multiple sclerosis. Trials of maintenance treatment with prednisolone and soluble aspirin. Lancet 1961;1(7169):127-9. - PubMed
Wolinsky 2018 {published data only}
-
- Wolinsky JS, Montalban X, Hauser SL, Giovannoni G, Vermersch P, Bernasconi C, Deol-Bhullar G, Garren H, Chin P, Belachew S, Kappos L. Evaluation of no evidence of progression or active disease (NEPAD) in patients with primary progressive multiple sclerosis in the ORATORIO trial. Ann Neurol 2018;84(4):527-536. [DOI: 10.1002/ana.25313] [PMID: ] - DOI - PMC - PubMed
References to ongoing studies
EUCTR2012‐003056‐36 {published data only}
-
- EUCTR2012-003056-36. https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_nu....
EUCTR2014‐003021‐18‐PL {published data only}
-
- A safety and efficacy study of BG00012 in slowing the progression of disability in patients with Secondary Progressive Multiple Sclerosis. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2014%E2%80%90003021....
EUCTR2018‐001511‐73‐ES {published data only}
-
- A Study to Evaluate the Efficacy and Safety of Ocrelizumab in Adults with Primary Progressive Multiple Sclerosis. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2018%E2%80%90001511....
EUCTR2018‐001511‐73‐GB {published data only}
-
- A phase IIIb multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of ocrelizumab in adults with primary progressive multiple sclerosis. https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_nu....
EUCTR2018‐005038‐39‐GB {published data only}
-
- A phase 2b study of Cladribine to halt deterioration in people with advanced multiple sclerosis. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2018%E2%80%90005038....
EUCTR2020‐002981‐15‐DK {published data only}
-
- Non-inferiority study of ocrelizumab and rituximab in active multiple sclerosis. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2020%E2%80%90002981....
IRCT20130812014333N125 {published data only}
-
- Comparison of effectiveness and complication of rituximab and fingolimod in improvement disability motion. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20130812014333N125.
NCT04035005 {published data only}
-
- A Study to Evaluate the Efficacy and Safety of Ocrelizumab in Adults With Primary Progressive Multiple Sclerosis. https://clinicaltrials.gov/study/NCT04035005.
NCT04688788 {published data only}
-
- Non-inferiority Study of Ocrelizumab and Rituximab in Active Multiple Sclerosis. https://clinicaltrials.gov/ct2/show/NCT04688788.
NCT04695080 {published data only}
-
- ChariotMS - Cladribine to Halt Deterioration in People With Advanced Multiple Sclerosis (ChariotMS). https://clinicaltrials.gov/show/NCT04695080.
Additional references
Aharoni 2014
-
- Aharoni R. Immunomodulation neuroprotection and remyelination. The fundamental therapeutic effects of glatiramer acetate: a critical review. Journal of Autoimmunity 2014;54:81-92. [PMID: ] - PubMed
Alonso‐Coello 2016a
-
- Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al, GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ 2016;353:i2016. [DOI: 10.1136/bmj.i2016] [PMID: ] - DOI - PubMed
Alonso‐Coello 2016b
-
- Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al, GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ 2016;353:i2089. [DOI: 10.1136/bmj.i2089] - DOI - PubMed
Awad 2009
Benedict 2017
Berntsson 2018
-
- Berntsson SG, Kristoffersson A, Boström I, Feresiadou A, Burman J, Landtblom AM. Rapidly increasing off-label use of rituximab in multiple sclerosis in Sweden - outlier or predecessor? Acta Neurologica Scandinavica 2018;138(4):327-31. - PubMed
Brancati 2021
Brennan 2020
Calabrese 2012
Capanna 2022
Carroll 2014
Chaimani 2013
Chitnis 2013
Chun 2010
Ciccone 2008
Cipriani 2013
-
- Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Annals of Internal Medicine 2013;159(2):130-7. - PubMed
Compston 2002
-
- Compston A, Coles A. Multiple sclerosis. Lancet 2002;359(9313):1221-31. - PubMed
Confavreux 2006
da Costa 2013
-
- da Costa BR, Nüesch E, Rutjes AW, Johnston BC, Reichenbach S, Trelle S, et al. Combining follow-up and change data is valid in meta-analyses of continuous outcomes: a meta-epidemiological study. Journal of Clinical Epidemiology 2013;66(8):847-55. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. - PubMed
Dutta 2014
EMA 2014
-
- European Medicines Agency. Nerventra. www.ema.europa.eu/en/medicines/human/EPAR/nerventra (accessed prior to 12 October 2022).
EMA 2015
-
- European Medicines Agency. Guideline on clinical investigation of medicinal products for the treatment of multiple sclerosis. www.ema.europa.eu/documents/scientific-guideline/guideline-clinical-inve... (accessed 26 December 2021).
EMA 2017
-
- European Medicines Agency. Mavenclad. www.ema.europa.eu/en/medicines/human/EPAR/mavenclad (accessed prior to 12 October 2022).
EMA 2018a
-
- European Medicines Agency. EMA recommends immediate suspension and recall of multiple sclerosis medicine Zinbryta. www.ema.europa.eu/en/news/ema-recommends-immediate-suspension-recall-mul... (accessed prior to 12 October 2022).
EMA 2018b
-
- European Medicines Agency. Ocrevus. www.ema.europa.eu/en/medicines/human/EPAR/ocrevus (accessed 31 July 2022).
EMA 2020
-
- European Medicines Agency. Mayzent. www.ema.europa.eu/en/medicines/human/EPAR/mayzent (accessed prior to 12 October 2022).
EMA 2021a
-
- European Medicines Agency. Zeposia. www.ema.europa.eu/en/medicines/human/EPAR/zeposia (accessed prior to 12 October 2022).
EMA 2021b
-
- European Medicines Agency. Ponvory. www.ema.europa.eu/en/medicines/human/EPAR/ponvory (accessed prior to 12 October 2022).
EMA 2021c
-
- European Medicines Agency. Vumerity. www.ema.europa.eu/en/medicines/human/EPAR/vumerity (accessed prior to 12 October 2022).
EMA 2021d
-
- European Medicines Agency. Kesimpta. www.ema.europa.eu/en/medicines/human/EPAR/kesimpta (accessed 31 July 2022).
FDA 2017
-
- US Food and Drug Administration. FDA approves new drug to treat multiple sclerosis. www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treat-... (accessed 31 July 2022).
FDA 2018
-
- US Food and Drug Administration. FDA working with manufacturers to withdraw Zinbryta from the market in the United States. www.fda.gov/drugs/drug-safety-and-availability/fda-working-manufacturers... (accessed prior to 12 October 2022).
FDA 2019a
-
- US Food and Drug Administration. FDA approves new oral treatment for multiple sclerosis. www.fda.gov/news-events/press-announcements/fda-approves-new-oral-treatm... (accessed prior to 12 October 2022).
FDA 2019b
-
- US Food and Drug Administration. FDA approves new oral drug to treat multiple sclerosis. www.fda.gov/news-events/press-announcements/fda-approves-new-oral-drug-t... (accessed prior to 12 October 2022).
FDA 2020
-
- US Food and Drug Administration. Novel drug approvals for 2020. www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-the... (accessed prior to 12 October 2022).
FDA 2021
-
- US Food and Drug Administration. Novel Drug Approvals for 2021. www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-the... (accessed prior to 12 October 2022).
Filippini 2013
-
- Filippini G, Del Giovane C, Vacchi L, D’Amico R, Di Pietrantonj C, Beecher D, et al. Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: CD008933. [DOI: 10.1002/14651858.CD008933.pub2] - DOI - PMC - PubMed
Fox 2004
-
- Fox E. Mechanism of action of mitoxantrone. Neurology 2004;12:15-8. [PMID: ] - PubMed
GBD 2019
Ghezzi 2018
Giovannoni 2022
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 3 February 2023. Hamilton (ON): McMaster University (developed by Evidence Prime), 2023. Available at gradepro.org.
Gronwall 1977
-
- Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Perceptual and Motor Skills 1977;44(2):367-73. - PubMed
Guyatt 2011
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence - indirectness. Journal of Clinical Epidemiology 2011;64(12):1303-10. - PubMed
Hauser 2020
Higgins 2003
Higgins 2012
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2019
-
- Higgins JP, Li T, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Higgins 2021
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Hu 2012
-
- Hu X, Miller L, Richman S, Hitchman S, Glick G, Liu S, et al. A novel PEGylated interferon beta-1a for multiple sclerosis: safety, pharmacology, and biology. Journal of Clinical Pharmacology 2012;52(6):798-808. [PMID: ] - PubMed
Hultcrantz 2017
Kappos 2011
-
- Kappos L, Calabresi PA, O'Connor P, Bar-Or A, Barkhof F, Yin M, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 2011;378(9805):1779-87. [PMID: ] - PubMed
Kieseier 2011
-
- Kieseier BC. The mechanism of action of interferon-β in relapsing multiple sclerosis. CNS Drugs 2011;25(6):491-502. [PMID: ] - PubMed
Kurtzke 1983
-
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33(11):1444-52. - PubMed
Lassmann 2012
-
- Lassmann H, Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nature Reviews. Neurology 2012;8(11):647-56. - PubMed
Laurson‐Doube 2021
Leist 2011
-
- Leist TP, Weissert R. Cladribine: mode of action and implications for treatment of multiple sclerosis. Clinical Neuropharmacology 2011;34(1):28-35. [PMID: ] - PubMed
Linker 2011
-
- Linker RA, Lee DH, Ryan S, Van Dam AM, Conrad R, Bista P, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011;134(3):678-92. [PMID: ] - PubMed
Lublin 2014
Lucchetta 2020
-
- Lucchetta RC, Oliveira ML, Bonetti AF, Fernandez-Llimos F, Wiens A. Outcome measures for disease-modifying therapies in relapsing multiple sclerosis randomized clinical trials: a scoping review protocol. JBI Evidence Synthesis 2020;18(8):1781-7. [DOI: 10.11124/JBISRIR-D-19-00178] [PMID: ] - DOI - PubMed
Lycke 2015
Massacesi 2002
-
- Massacesi L. Compartmentalization of the immune response in the central nervous system and natural history of multiple sclerosis. Implications for therapy. Clinical Neurology and Neurosurgery 2002;104(3):177-81. - PubMed
McDonald 2001
-
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Annals of Neurology 2001;50(1):121-7. - PubMed
Meinl 2008
-
- Meinl E, Krumbholz M, Derfuss T, Junker A, Hohlfeld R. Compartmentalization of inflammation in the CNS: a major mechanism driving progressive multiple sclerosis. Journal of the Neurological Sciences 2008;274(1-2):42-4. - PubMed
Meyer‐Moock 2014
Microsoft Excel [Computer program]
-
- Microsoft Excel. Version 2405. Microsoft Corporation, 2024. Available from https://office.microsoft.com/excel.
Millard 2011
Morgano 2022
-
- Morgano GP, Mbuagbaw L, Santesso N, Xie F, Brozek JL, Siebert U, et al. Defining decision thresholds for judgments on health benefits and harms using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Evidence to Decision (EtD) frameworks: a protocol for a randomised methodological study (GRADE-THRESHOLD). BMJ Open 2022;12(3):e053246. - PMC - PubMed
Oh 2013
Peters 2008
-
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61(10):991-6. - PubMed
Puhan 2014
-
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ (Clinical Research Edition) 2014;349:g5630. - PubMed
Pöhlau 2007
-
- Pöhlau D, Przuntek H, Sailer M, Bethke F, Koehler J, König N, et al. Intravenous immunoglobulin in primary and secondary chronic progressive multiple sclerosis: a randomized placebo controlled multicentre study. Multiple Sclerosis (Houndmills, Basingstoke, England) 2007;13(9):1107-17. - PubMed
Reich 2018
RevMan 2024 [Computer program]
-
- Review Manager (RevMan). Version 7.2.0. The Cochrane Collaboration, 2024. Available at revman.cochrane.org.
Rhodes 2015
S.O.S.MS Project 2020
-
- Daniels K, Frequin S, Wees PJ, de Garde EM, Biesma DH, SOS MS Expert Panel, et al. Development of the international, multidisciplinary, patient-relevant standard outcome set for Multiple Sclerosis: the S.O.S.MS project. spem.pt/wp-content/uploads/2021/03/S.O.S.MS_ACTRIMS_KDaniels_18-12-2020-... (accessed prior to 12 October 2022).
Salanti 2011
-
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163-71. - PubMed
Salanti 2012
-
- Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80-97. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.gradepro.org/app/handbook/handbook.html.
Schünemann 2022
-
- Schünemann HJ, Neumann I, Hultcrantz M, Brignardello-Petersen R, Zeng L, Murad MH, et al. GRADE guidance 35: update on rating imprecision for assessing contextualized certainty of evidence and making decisions. Journal of Clinical Epidemiology 2022;150:225-242. - PubMed
Silva 2022
Soelberg Sorensen 2008
Stata [Computer program]
-
- Stata. Version 17. College Station, TX: StataCorp, 2024. Available from https://www.stata.com.
Tramacere 2015
-
- Tramacere I, Del Giovane C, Salanti G, D'Amico R, Filippini G. Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD011381. [DOI: 10.1002/14651858.CD011381.pub2] - DOI - PMC - PubMed
Turner 2012
Veroniki 2013
Walton 2020
Whitaker 1995
White 2011
-
- White IR. Multivariate random-effects meta-regression: updates to mvmeta. Stata Journal 2011;11(2):255-70. [DOI: 10.1177/1536867X1101100206] - DOI
Yepes‐Nuñez 2019
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

