Testosterone treatment impacts the intestinal microbiome of transgender individuals
- PMID: 39254049
- PMCID: PMC11520287
- DOI: 10.1128/msphere.00557-24
Testosterone treatment impacts the intestinal microbiome of transgender individuals
Abstract
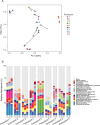
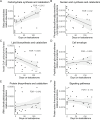
Medical modulation of sex hormone levels is a cornerstone of treatment for many conditions that impact well-being, including cancer, fertility/infertility, gender dysphoria, and chronic metabolic diseases such as diabetes and obesity. The microbial residents of the intestine, known as the microbiota, interact with sex hormones in the intestine, and there is correlative evidence that this interaction is bidirectional. Based on these published findings, we hypothesized that transgender individuals receiving exogenous testosterone as part of their gender-affirming medical treatment might undergo changes in their intestinal microbiome. To test this, we collected 26 stool samples from nine individuals before and up to 8 months after initiation of treatment with exogenous testosterone and subjected these samples to metagenomic analysis. While no species were significantly associated with the duration of testosterone therapy, pathways that generate glutamate increased in abundance, while those that consume glutamate decreased. Glutamate is a precursor of arginine, and testosterone is known to increase levels of arginine and its metabolites in the plasma. We hypothesize that testosterone increases the uptake of glutamate by enterocytes, thus decreasing access of the microbiota to this amino acid. While this pilot study establishes the impact of testosterone therapy on the intestinal microbiome, a more comprehensive study is necessary to establish the impact of testosterone-driven metagenomic shifts on the stool metatranscriptome, the stool metabolome, and the plasma metabolome.IMPORTANCEThe human intestine is inhabited by a large community of microbes known as the microbiome. Members of the microbiome consume the diet along with their human host. Thus, the metabolomes of the host and microbe are intricately linked. Testosterone alters the plasma metabolome. In particular, plasma levels of arginine and its metabolites and testosterone are positively correlated. To investigate the impact of exogenous testosterone on the microbiome, we analyzed the stool metagenomes of transgender individuals before and after the initiation of testosterone treatment. In this pilot project, we found a modest impact on the microbiome community structure but an increase in the abundance of metabolic pathways that generate glutamate and spare glutamate consumption. We propose that the host uses glutamate to generate arginine, decreasing the amount available for the microbiome.
Keywords: arginine metabolism; gut microbiome; human microbiome; metagenomics; testosterone; transgender.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ter Horst R, van den Munckhof ICL, Schraa K, Aguirre-Gamboa R, Jaeger M, Smeekens SP, Brand T, Lemmers H, Dijkstra H, Galesloot TE, de Graaf J, Xavier RJ, Li Y, Joosten LAB, Rutten JHW, Netea MG, Riksen NP. 2020. Sex-specific regulation of inflammation and metabolic syndrome in obesity. Arterioscler Thromb Vasc Biol 40:1787–1800. doi:10.1161/ATVBAHA.120.314508 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

