Hypertension and cerebral blood flow in the development of Alzheimer's disease
- PMID: 39254220
- PMCID: PMC11567827
- DOI: 10.1002/alz.14233
Hypertension and cerebral blood flow in the development of Alzheimer's disease
Abstract
Introduction: We investigated the interactive associations between amyloid and hypertension on the entorhinal cortex (EC) tau and atrophy and the role of cerebral blood flow (CBF) as a shared mechanism by which amyloid and hypertension contribute to EC tau and regional white matter hyperintensities (WMHs).
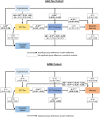
Methods: We analyzed data from older adults without dementia participating in the Add-Tau study (NCT02958670, n = 138) or Alzheimer's Disease Neuroimaging Initiative (ADNI) (n = 523) who had available amyloid-positron emission tomography (PET), tau-PET, fluid-attenuated inversion recovery (FLAIR), and T1-weighted magnetic resonance imaging (MRI). A subsample in both cohorts had available arterial spin labeling (ASL) MRI (Add-Tau: n = 78; ADNI: n = 89).
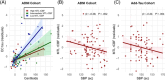
Results: The detrimental effects of hypertension on AD pathology and EC thickness were more pronounced in the Add-Tau cohort. Increased amyloid burden was associated with decreased occipital gray matter CBF in the ADNI cohort. In both cohorts, lower regional gray matter CBF was associated with higher EC tau and posterior WMH burden.
Discussion: Reduced cerebral perfusion may be one common mechanism through which hypertension and amyloid are related to increased EC tau and WMH volume.
Highlights: Hypertension is associated with increased entorhinal cortex (EC) tau, particularly in the presence of amyloid. Decreased cortical cerebral blood flow (CBF) is associated with higher regional white matter hyperintensity volume. Increasing amyloid burden is associated with decreasing CBF in the occipital lobe. MTL CBF and amyloid are synergistically associated with EC tau.
Keywords: Alzheimer's disease; amyloid pathology; arterial spin labeling; cerebral perfusion; regional white matter hyperintensities; small vessel disease; tau pathology.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Christoph Hock and Roger M. Nitsch are employees and shareholders of Neurimmune AG, Switzerland. Dario Bachmann, Antje Saake, Sandro Studer, Andreas Buchmann, Katrin Rauen, Esmeralda Gruber, Lars Michels, Anton Gietl, and Valerie Treyer declare no relevant conflicts of interest. Author disclosures are available in the supporting information.
Figures


References
-
- Braak H, Braak E. Frequency of stages of Alzheimer‐related lesions in different age categories. Neurobiol Aging. 1997;18(4):351‐357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

