Quality-of-life outcomes from NRG Oncology NSABP B-39/RTOG 0413: whole-breast irradiation vs accelerated partial-breast irradiation after breast-conserving surgery
- PMID: 39254630
- PMCID: PMC11717410
- DOI: 10.1093/jnci/djae219
Quality-of-life outcomes from NRG Oncology NSABP B-39/RTOG 0413: whole-breast irradiation vs accelerated partial-breast irradiation after breast-conserving surgery
Abstract
Background: NRG Oncology NSABP B-39/RTOG 0413 compared whole-breast irradiation (WBI) to accelerated partial-breast irradiation (APBI). APBI was not equivalent to WBI in local tumor control. Secondary outcome was quality of life (QOL).
Methods: The QOL sub-study used validated self-report questionnaires, including the Breast Cancer Treatment Outcome Scale (BCTOS) and the 36-Item Short Form Health Survey (SF-36) vitality scale. Assessments occurred before random assignment, at treatment completion (chemotherapy or radiotherapy), 4 weeks later, at 6, 12, 24, and 36 months. Primary aims: cosmesis change equivalency (baseline to 3 years; a priori margin of equivalence 0.4 standard deviations) and fatigue change superiority (baseline to end of treatment [EOT]) for APBI vs WBI, by patient groups treated with or without chemotherapy when appropriate.
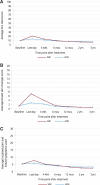
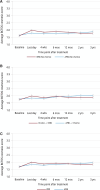
Results: From March 21, 2005 to May 25, 2009, 975 patients enrolled in this sub-study; 950 had follow-up data. APBI had 3-year cosmesis equivalent to WBI (95% CI = -0.0001 to -0.16; equivalence margin -0.22 to -0.22) in all patients. The APBI group without chemotherapy had less EOT fatigue (P = .011; mean score APBI 63 vs WBI 59); the APBI group receiving chemotherapy had worse EOT fatigue (P = .011; APBI 43 vs WBI 49). The APBI group reported less pain (BCTOS) at EOT (WBI 2.29 vs APBI 1.97) but worse pain at 3 years (WBI 1.62 vs APBI 1.71). APBI patients reported greater convenience of care than with WBI and reported less symptom severity at EOT and 4 weeks later.
Conclusion: Cosmetic outcomes were similar for the APBI and WBI groups, with small statistically significant differences in other outcomes that varied over time. Differences in fatigue and other symptoms appeared to resolve by ≥6 months. APBI may be preferred by some patients, for whom extended treatment is burdensome.
Clinicaltrials.gov: NCT00103181.
© The Author(s) 2024. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
All authors report NCI grants: U10CA180868, U10CA180822, and UG1CA189867, paid to institution.
Patricia A. Ganz, MD—Reports: Royalty for UpToDate Section Editor; and consulting fees for Genentech Roche (for QOL in a clinical trial) and InformedDNA (Scientific Advisory Board).
Rachel A. Rabinovitch, MD—Reports: Payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events, unrelated to the content of this article, from Miami Breast Cancer Conference 2023 as an invited speaker and Northwell Health Cancer Institute 2022 as a Grand Rounds Invited Speaker; support for attending meetings and/or travel, unrelated to the content of this article, from Miami Breast Cancer Conference 2023 as an invited speaker, Northwell Health Cancer Institute 2022 as a Grand Rounds Invited Speaker, and NRG Oncology, twice annually, as the Vice-Chair of the Membership Committee; and no stocks/stock options related to this article.
David S. Parda, MD—Reports: Operations support from NRG for study management and oversight duties.
Michael F. Scheier, PhD—Reports: Being a paid consultant for NSABP, royalties or licenses for Pearson Publishing (for Personality Textbook) and Cambridge University Press (for book written); Honorarium (for speaking at retirement of friend); and stock or options with Apple and General Electric.
Henry M. Kuerer, MD, PhD—Reports: Funding support from American College of Surgeons Oncology Group and Alliance for Clinical Trials in Oncology; support for attending meetings and/or travel: Alliance for Clinical Trials in Oncology; leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid: Chair Publications Committee, American Society of Breast Surgeons, and paid editor, NEJM Group, Inc.
Eleftherios P. Mamounas, MD—Reports: Consulting fees from Genentech, Merck, Exact Sciences, Biotheranostics, Delphi Diagnostics, Novartis, Tersera, and Sanofi-Genzyme; payments or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Genentech, Merck, and Exact Sciences; support for attending a meeting or travel from Alapharma; and stock or stock options with Moderna.
Harry D. Bear, MD—Reports: Stock with AbbVie and Pfizer, and Viatris, unrelated to this work.
Ivy A. Petersen, MD—Reports: Support for attending meetings and/or travel from NRG Oncology: payments to me as co-chair of the Radiation Oncology Committee.
Walter J. Curran, Jr, MD: Reports: Participation in a Data Monitoring Committee with Bristol Meyers Squibb and Astra Zeneca; stock/stock options with Arterra, Lumonus, and GenesisCare.
Norman Wolmark, MD: Reports: the Breast Cancer Research Foundation (BCRF); the Susan G. Komen Award for Scientific Distinction in Clinical Research; and the American Surgical Association Medallion for Advancement of Surgical Care.
All other authors declare no other potential conflicts of interest.
Figures


References
-
- Agarwal S, Pappas L, Neumayer L, Kokeny K, Agarwal J.. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg. 2014;149(3):267-274. - PubMed
-
- van Maaren MC, de Munck L, de Bock GH, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol. 2016;17(8):1158-1170. - PubMed
-
- Whelan TJ, Julian JA, Berrang TS, et al. RAPID Trial Investigators External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): a randomised controlled trial. Lancet. 2019;394(10215):2165-2172. - PubMed
-
- Schäfer R, Strnad V, Polgár C, et al. ; Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO): Quality-of-life results for accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation in early breast cancer after breast-conserving surgery (GEC-ESTRO): 5-year results of a randomised, phase 3 trial. Lancet Oncol. 2018;19(6):834-844. - PubMed
-
- Meattini I, Marrazzo L, Saieva C, et al. Accelerated partial-breast irradiation compared with whole-breast irradiation for early breast cancer: long-term results of the randomized phase III APBI-IMRT-Florence trial. J Clin Oncol. 2020;38(35):4175-4183. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

