Impact of Previous Alopecia Areata Treatment on Efficacy Responses up to Week 48 Following Ritlecitinib Treatment: A Post Hoc Analysis
- PMID: 39254890
- PMCID: PMC11480292
- DOI: 10.1007/s13555-024-01260-7
Impact of Previous Alopecia Areata Treatment on Efficacy Responses up to Week 48 Following Ritlecitinib Treatment: A Post Hoc Analysis
Abstract
Introduction: Patients with alopecia areata (AA) may have received several therapies for management of AA during their lives. In the ALLEGRO phase 2b/3 (NCT03732807) study, the oral JAK3/TEC family kinase inhibitor ritlecitinib demonstrated efficacy and an acceptable safety profile in patients aged ≥ 12 years with AA and ≥ 50% scalp hair loss. This post hoc analysis investigated associations between prior use of AA therapies and Severity of Alopecia Tool (SALT) responses in patients receiving ritlecitinib for AA.
Methods: Patients receiving ritlecitinib 30 mg or 50 mg once daily with or without an initial 4-week 200-mg daily loading dose were grouped by previous exposure to AA treatments, including topicals, intralesional corticosteroids (ILCS), topical immunotherapy, and systemic immunosuppressants or any prior AA treatment. Multivariable logistic regression analyses evaluated the association between response based on a SALT score of ≤ 20 and any prior treatment for AA at weeks 24 and 48.
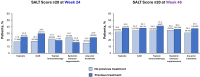
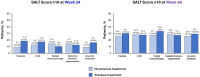
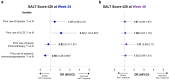
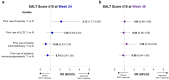
Results: Of 522 patients, 360 (69.0%) had previous exposure to any AA treatment. At Week 24, SALT ≤ 20 response was positively associated with prior use of ILCS (odds ratio [OR], 2.12; 95% confidence interval [CI], 1.23-3.65; P < 0.05) and negatively associated with prior use of systemic immunosuppressants (OR 0.50; 95% CI 0.28-0.88; P < 0.05). Prior use of topicals or topical immunotherapy was not associated with SALT ≤ 20 response at Week 24. By Week 48, no association was identified between SALT ≤ 20 response and prior use of topicals, ILCS, topical immunosuppressants, or systemic immunosuppressants (all P > 0.05). Previous exposure to any AA therapy was not associated with SALT ≤ 20 response at weeks 24 or 48 (all P > 0.05).
Conclusions: Prior AA treatment history had no effect on longer-term treatment response to ritlecitinib.
Trial registration number: NCT03732807.
Keywords: Alopecia areata; JAK inhibitor; Post hoc analysis; Ritlecitinib; SALT; Treatment.
© 2024. The Author(s).
Conflict of interest statement
Jennifer Fu has received consultation fees from Pfizer. Alexander Egeberg has received research funding from Pfizer, Eli Lilly, Novartis, Bristol Myers Squibb, AbbVie, Janssen, and Boehringer Ingelheim and has received honoraria for work as a consultant and/or speaker from AbbVie, Almirall, LEO Pharma, Zuellig Pharma, Galapagos NV, SUN Pharmaceuticals, Samsung Bioepis, Pfizer, Eli Lilly, Novartis, Union Therapeutics, Galderma, Dermavant, UCB, Mylan, Bristol Myers Squibb, McNeil Consumer Healthcare, Horizon Therapeutics, Boehringer Ingelheim, and Janssen. Susan Holmes is an investigator for Pfizer and has undertaken paid consultancy work for Pfizer. Sergio Vano-Galvan has received research funding and honoraria for work as a consultant and/or speaker from Pfizer and Eli Lilly. Martin Steinhoff has received honoraria or investigative or consultation fees from or was an investigator for AbbVie, Almirall, Avon, Algorithm, Allergan, Bayer Health, Baiersdorf, Bristol Myers Squibb, Celgene, Chugai, Ducray, Eli Lilly, Galderma, Genentech, GSK, Incyte, Janssen, Johnson & Johnson, Kiniksa, LEO Pharma, L’Oreal, Maruho, Menlo Therapeutics, Mitsubishi, Novartis, Pfizer, Pierre-Fabre, Qatar Pharma, Regeneron, Sanofi, Toray, Trevi, Vertex, and ZymoGenetics. Roger Edwards is an employee of Health Services Consulting Corporation and received consultancy fees from Pfizer in connection with this study. Gianluca Bonfanti is an employee of Engineering Ingegneria Informatica, which is a paid subcontractor to Health Services Consulting Corporation. Ranjit Nagra, Robert Wolk, Helen Tran, and Ernest Law are employees of, and hold stock or stock options in, Pfizer.
Figures

References
-
- Islam N, Leung PS, Huntley AC, Gershwin ME. The autoimmune basis of alopecia areata: a comprehensive review. Autoimmun Rev. 2015;14(2):81–9. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

