A Mathematical Model of Interstitial Fluid Flow and Retinal Tissue Deformation in Macular Edema
- PMID: 39254963
- PMCID: PMC11401122
- DOI: 10.1167/iovs.65.11.19
A Mathematical Model of Interstitial Fluid Flow and Retinal Tissue Deformation in Macular Edema
Abstract
Purpose: This study aims to develop a mathematical model to elucidate fluid circulation in the retina, focusing on the movement of interstitial fluid (comprising water and albumin) to understand the mechanisms underlying exudative macular edema (EME).
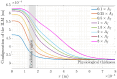
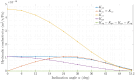
Methods: The model integrates physiological factors such as retinal pigment epithelium (RPE) pumping, osmotic pressure gradients, and tissue deformation. It accounts for spatial variability in hydraulic conductivity (HC) across the retina and incorporates the structural role of Müller cells (MCs) in maintaining retinal stability.
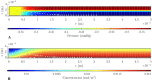
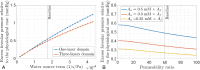
Results: The model predicts that tissue deformation is maximal at the center of the fovea despite fluid exudation from blood capillaries occurring elsewhere, aligning with clinical observations. Additionally, the model suggests that spatial variability in HC across the thickness of the retina plays a protective role against fluid accumulation in the fovea.
Conclusions: Despite inherent simplifications and uncertainties in parameter values, this study represents a step toward understanding the pathophysiology of EME. The findings provide insights into the mechanisms underlying fluid dynamics in the retina and fluid accumulation in the foveal region, showing that the specific conformation of Müller cells is likely to play a key role.
Conflict of interest statement
Disclosure:
Figures




References
-
- Govetto A, Hubschman J-P, Sarraf D, et al. .. The role of Müller cells in tractional macular disorders: an optical coherence tomography study and physical model of mechanical force transmission. Br J Ophthalmol. 2020; 104(4): 466–472. - PubMed
-
- Daruich A, Matet A, Moulin A, et al. .. Mechanisms of macular edema: beyond the surface. Prog Retin Eye Res. 2018; 63: 20–68. - PubMed
-
- Dvoriashyna M, Foss AJE, Gaffney EA, Jensen OE, Repetto R. Osmotic and electroosmotic fluid transport across the retinal pigment epithelium: a mathematical model. J Theor Biol. 2018; 456: 233–248. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

