Antibiotic use during influenza infection augments lung eosinophils that impair immunity against secondary bacterial pneumonia
- PMID: 39255040
- PMCID: PMC11527449
- DOI: 10.1172/JCI180986
Antibiotic use during influenza infection augments lung eosinophils that impair immunity against secondary bacterial pneumonia
Abstract
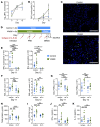
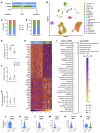
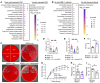
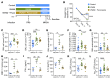
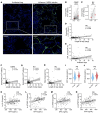
A leading cause of mortality after influenza infection is the development of a secondary bacterial pneumonia. In the absence of a bacterial superinfection, prescribing antibacterial therapies is not indicated but has become a common clinical practice for those presenting with a respiratory viral illness. In a murine model, we found that antibiotic use during influenza infection impaired the lung innate immunologic defenses toward a secondary challenge with methicillin-resistant Staphylococcus aureus (MRSA). Antibiotics augment lung eosinophils, which have inhibitory effects on macrophage function through the release of major basic protein. Moreover, we demonstrated that antibiotic treatment during influenza infection caused a fungal dysbiosis that drove lung eosinophilia and impaired MRSA clearance. Finally, we evaluated 3 cohorts of hospitalized patients and found that eosinophils positively correlated with antibiotic use, systemic inflammation, and worsened outcomes. Altogether, our work demonstrates a detrimental effect of antibiotic treatment during influenza infection that has harmful immunologic consequences via recruitment of eosinophils to the lungs, thereby increasing the risk of developing a secondary bacterial infection.
Keywords: Bacterial infections; Infectious disease; Influenza; Innate immunity; Pulmonology.
Figures


References
MeSH terms
Substances
Grants and funding
- R03 HL162655/HL/NHLBI NIH HHS/United States
- F32 HL162377/HL/NHLBI NIH HHS/United States
- K23 HL169815/HL/NHLBI NIH HHS/United States
- U19 AI135964/AI/NIAID NIH HHS/United States
- P01 HL154998/HL/NHLBI NIH HHS/United States
- U01 TR003528/TR/NCATS NIH HHS/United States
- KL2 TR001882/TR/NCATS NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- R01 HL164177/HL/NHLBI NIH HHS/United States
- R01 HL159953/HL/NHLBI NIH HHS/United States
- P01 HL114453/HL/NHLBI NIH HHS/United States
- R01 LM013337/LM/NLM NIH HHS/United States
- R01 HL155759/HL/NHLBI NIH HHS/United States
- R01 HL163646/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

