Population pharmacokinetics of unbound cefazolin in infected hospitalized patients requiring intermittent high-flux haemodialysis: can a three-times-weekly post-dialysis dosing regimen provide optimal treatment?
- PMID: 39255245
- PMCID: PMC11531813
- DOI: 10.1093/jac/dkae318
Population pharmacokinetics of unbound cefazolin in infected hospitalized patients requiring intermittent high-flux haemodialysis: can a three-times-weekly post-dialysis dosing regimen provide optimal treatment?
Abstract
Objectives: To describe the population pharmacokinetics of cefazolin in infected hospitalized patients requiring intermittent haemodialysis (IHD).
Methods: This prospective population pharmacokinetic study was conducted in IHD patients prescribed cefazolin 2 g three times weekly. Plasma samples were collected at prespecified timepoints and assayed for total and unbound concentrations using validated LC. Pharmacokinetic modelling and dosing simulations were performed using Pmetrics®. PTA in plasma suitable for MSSA (unbound trough concentrations of ≥2 mg/L for the final 24 h of a 72 h interval) were simulated for different dosing regimens. A PTA of ≥95% was deemed acceptable.
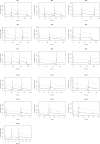
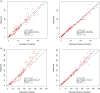
Results: A total of 260 cefazolin concentrations (130 total, 130 unbound) were collected from 16 patients (14 female) with a median age of 51 years. The median (IQR) pre-dialysis unbound cefazolin concentration for a 3 day dose interval trough was 17.7 (13.5-31.4) mg/L. The median (IQR) unbound fraction was 0.38 (0.32-0.46). The lowest pre-dialysis unbound concentration was 9.1 mg/L. A two-compartment model with a complex protein-binding component adequately described the data. The mean unbound cefazolin CL during IHD was 16.4 ± 4.26 L/h, compared with 0.40 ± 0.19 L/h when dialysis was off. Duration of time on haemodialysis (TOH) was the only covariate supported in the final model. The 2 g three-times-weekly regimen was associated with a PTA of 99.7% on dosing simulations to maintain unbound concentrations of ≥2 mg/L with TOH of 6 months. The 1 g three-times-weekly post-dialysis was associated with a PTA of 95.4%.
Conclusions: A 2 g three-times-weekly post-dialysis cefazolin regimen is supported for MSSA infections.
© The Author(s) 2024. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

