Naldemedine for Opioid-Induced Constipation in Patients With Cancer: A Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial
- PMID: 39255425
- PMCID: PMC11637578
- DOI: 10.1200/JCO.24.00381
Naldemedine for Opioid-Induced Constipation in Patients With Cancer: A Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial
Erratum in
-
Erratum: Naldemedine for Opioid-Induced Constipation in Patients With Cancer: A Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial.J Clin Oncol. 2025 May;43(13):1615. doi: 10.1200/JCO-25-00567. Epub 2025 Mar 21. J Clin Oncol. 2025. PMID: 40117532 Free PMC article. No abstract available.
Abstract
Purpose: Opioid-induced constipation is the most frequent and non-self-limiting adverse effect of opioid analgesia, reducing adherence and interfering with pain relief. This clinical trial aimed to clarify the preventive effect of naldemedine versus placebo for constipation in patients with cancer starting regularly dosed strong opioids therapy.
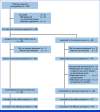
Methods: This multicenter, double-blinded, randomized, placebo-controlled, confirmatory trial was conducted between July 2021 and May 2023 at four academic hospitals in Japan (Japan Registry of Clinical Trials identifier: jRCTs031200397). Patients with cancer starting a first-time regularly dosed strong opioid for cancer pain and age 20+ years were included. Eligible patients were randomly assigned to the naldemedine (Symproic 0.2 mg) or placebo group in a 1:1 ratio for 14 days with protocol treatment. The primary end point was the proportion of patients with a Bowel Function Index (BFI) of <28.8 on day 14. The secondary end points included frequency of spontaneous bowel movements (SBM), quality of life (QOL), and frequency of opioid-induced nausea and vomiting (OINV).
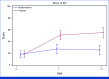
Results: Of the 103 patients assessed for eligibility, 99 received either naldemedine (n = 49) or placebo (n = 50). A BFI of <28.8 on day 14 was significantly more likely to occur in the naldemedine group (64.6%; 95% CI, 51.1 to 78.1) versus placebo (17.0%; 95% CI, 6.3 to 27.8), and the difference between groups was 47.6% (95% CI, 30.3 to 64.8; P < .0001). The frequency of SBM, QOL, and the severity of OINV were nominally significant in the naldemedine group than in the control group.
Conclusion: Naldemedine prevented constipation and improved constipation-related QOL, with possible preventive effect on OINV in patients with cancer starting regularly dosed opioids therapy.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
-
- Caraceni A, Hanks G, Kaasa S, et al. : Use of opioid analgesics in the treatment of cancer pain: Evidence-based recommendations from the EAPC. Lancet Oncol 13:e58-e68, 2012 - PubMed
-
- National Comprehensive Cancer Network : NCCN Clinical Practice Guideline in Oncology: Palliative care. V.2.2023. 2023. https://www.nccn.org - PubMed
-
- Paice JA, Bohlke K, Barton D, et al. : Use of opioids for adults with pain from cancer or cancer treatment: ASCO Guideline. J Clin Oncol 41:914-930, 2023 - PubMed
-
- Jesuyajolu DA, Abubakar AK, Kowe T, et al. : The management of opioid-induced constipation in cancer and advanced illness: A meta-analysis. J Pain Symptom Manage 67:e285-e297, 2024 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical