Final Overall Survival Analysis of S1500: A Randomized, Phase II Study Comparing Sunitinib With Cabozantinib, Crizotinib, and Savolitinib in Advanced Papillary Renal Cell Carcinoma
- PMID: 39255440
- PMCID: PMC11575905
- DOI: 10.1200/JCO.24.00767
Final Overall Survival Analysis of S1500: A Randomized, Phase II Study Comparing Sunitinib With Cabozantinib, Crizotinib, and Savolitinib in Advanced Papillary Renal Cell Carcinoma
Abstract
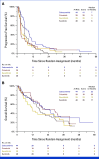
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.Mesenchymal-epithelial transition (MET) signaling pathway plays a role in the pathogenesis of selected patients with papillary renal cell carcinoma (PRCC). In the phase II PAPMET trial (ClinicalTrials.gov identifier: NCT02761057), cabozantinib significantly prolonged progression-free survival and improved objective response rate compared with sunitinib in patients with advanced PRCC. Here, we present the final overall survival (OS) analysis. In this multicenter, randomized phase II, open-label trial, 147 patients with advanced PRCC who have received up to one previous therapy (excluding vascular endothelial growth factor-directed agents) were assigned to sunitinib, cabozantinib, crizotinib, or savolitinib. Ultimately, savolitinib and crizotinib arms were closed because of futility. With a median follow-up of 17.5 months, the median OS was 21.5 months (95% CI, 12.0 to 28.1) with cabozantinib and 17.3 months (95% CI, 12.8 to 21.8) with sunitinib (hazard ratio, 0.83; 95% CI, 0.51 to 1.36; P = .46). The OS landmark estimates for cabozantinib and sunitinib were 50% versus 39% at 24 months and 32% versus 28% at 36 months. In conclusion, we observed no significant difference in OS across treatment arms. Although cabozantinib represents a well-supported option for advanced PRCC, the lack of survival benefit underscores the need to develop novel therapies for this disease.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
References
-
- Sweeney PL, Jang A, Halat SK, et al. Advanced papillary renal cell carcinoma: Epidemiology, genomic drivers, current therapies, and ongoing trials. Cancer Treat Res Commun. 2022;33:100639. - PubMed
-
- Dizman N, Arslan ZE, Feng M, et al. Sequencing therapies for metastatic renal cell carcinoma. Urol Clin North Am. 2020;47:305–318. - PubMed
-
- Pal SK, Ali SM, Yakirevich E, et al. Characterization of clinical cases of advanced papillary renal cell carcinoma via comprehensive genomic profiling. Eur Urol. 2018;73:71–78. - PubMed
-
- Albiges L, Guegan J, Le Formal A, et al. MET is a potential target across all papillary renal cell carcinomas: Result from a large molecular study of pRCC with CGH array and matching gene expression array. Clin Cancer Res. 2014;20:3411–3421. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous