HAF prevents hepatocyte apoptosis and progression to MASH and HCC through transcriptional regulation of the NF-κB pathway
- PMID: 39255518
- PMCID: PMC12266801
- DOI: 10.1097/HEP.0000000000001070
HAF prevents hepatocyte apoptosis and progression to MASH and HCC through transcriptional regulation of the NF-κB pathway
Abstract
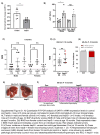
Background and aims: HCC incidence is increasing worldwide due to the obesity epidemic, which drives metabolic dysfunction-associated steatohepatitis (MASH) that can lead to HCC. However, the molecular pathways driving MASH-HCC are poorly understood. We have previously reported that male mice with haploinsufficiency of hypoxia-associated factor (HAF) ( SART1+/ - ) spontaneously develop MASH-HCC. However, the cell type(s) responsible for HCC associated with HAF loss are unclear.
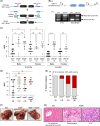
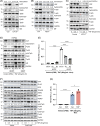
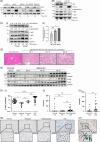
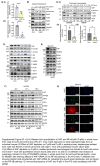
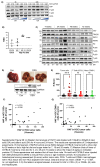
Approach and results: We generated SART1 -floxed mice, which were crossed with mice expressing Cre recombinase within hepatocytes (Alb-Cre; hepS -/- ) or myeloid cells (LysM-Cre, macS -/- ). HepS - / - mice (both male and female) developed HCC associated with profound inflammatory and lipid dysregulation, suggesting that HAF protects against HCC primarily within hepatocytes. HAF-deficient hepatocytes showed decreased P-p65 and P-p50 in many components of the NF-κB pathway, which was recapitulated using HAF small interfering RNA in vitro. HAF depletion also triggered apoptosis, suggesting that HAF protects against HCC by suppressing hepatocyte apoptosis. We show that HAF regulates NF-κB activity by regulating the transcription of TRADD and RIPK1 . Mice fed a high-fat diet showed marked suppression of HAF, P-p65, and TRADD within their livers after 26 weeks but showed profound upregulation of these proteins after 40 weeks, implicating deregulation of the HAF-NF-κB axis in the progression to MASH. In humans, HAF was significantly decreased in livers with simple steatosis but significantly increased in HCC compared with normal liver.
Conclusions: HAF is a novel transcriptional regulator of the NF-κB pathway and is a key determinant of cell fate during progression to MASH and MASH-HCC.
Keywords: HAF; HCC; MASH; NF-κB; apoptosis; hepatocyte.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Scott. A. Summers owns stock in Centaurus. Mei Yee Koh received grants, is employed by, owns stock, and holds intellectual property rights with Kuda. The remaining authors have no conflicts to report.
Figures







Update of
-
HAF Prevents Hepatocyte Apoptosis and Hepatocellular Carcinoma through Transcriptional Regulation of the NF-κB pathway.bioRxiv [Preprint]. 2024 Jan 11:2024.01.09.574894. doi: 10.1101/2024.01.09.574894. bioRxiv. 2024. Update in: Hepatology. 2025 Aug 1;82(2):438-453. doi: 10.1097/HEP.0000000000001070. PMID: 38260413 Free PMC article. Updated. Preprint.
References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–263. - PubMed
-
- Llovet JM, Villanueva A, Marrero JA, Schwartz M, Meyer T, Galle PR, et al. Trial design and endpoints in hepatocellular carcinoma: AASLD Consensus Conference. Hepatology. 2021;73 Suppl 1(Suppl 1):158–191. - PubMed
-
- Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

