Trastuzumab deruxtecan in patients with previously treated HER2-low advanced breast cancer and active brain metastases: the DEBBRAH trial
- PMID: 39255534
- PMCID: PMC11415677
- DOI: 10.1016/j.esmoop.2024.103699
Trastuzumab deruxtecan in patients with previously treated HER2-low advanced breast cancer and active brain metastases: the DEBBRAH trial
Abstract
Background: Trastuzumab deruxtecan (T-DXd) is approved for human epidermal growth factor receptor 2 (HER2)-positive and HER2-low advanced breast cancer (ABC). T-DXd has shown encouraging intracranial activity in HER2-positive ABC patients with stable or active brain metastases (BMs); however, its efficacy in patients with HER2-low ABC with BMs is not well established yet.
Methods: DEBBRAH is a single-arm, five-cohort, phase II study evaluating T-DXd in patients with central nervous system involvement from HER2-positive and HER2-low ABC. Here, we report results from patients with heavily pretreated HER2-low ABC and active BMs, enrolled in cohorts 2 (n = 6, asymptomatic untreated BMs) and 4 (n = 6, progressing BMs after local therapy). Patients received 5.4 mg/kg T-DXd intravenously once every 21 days. The primary endpoint was intracranial objective response rate per Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) for both cohorts.
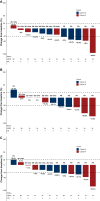
Results: Intracranial objective response rate per RANO-BM was 50.0% [3/6 patients; 95% confidence interval (CI) 11.8% to 88.2%] and 33.3% [2/6 patients; 95% CI 4.3% to 77.7%; P = 0.033 (one-sided)] in cohorts 2 and 4, respectively. All responders had partial responses. Median time to intracranial response was 2.3 months (range, 1.5-4.0 months) and median duration of intracranial response was 7.2 months (range, 2.8-16.8 months). Median progression-free survival per RECIST v.1.1. was 5.4 months (95% CI 4.1-10.0 months). Treatment-emergent adverse events occurred in all patients included (16.7% grade 3). Three patients (25.0%) had grade 1 interstitial lung disease/pneumonitis.
Conclusions: T-DXd demonstrated promising intracranial activity in pretreated HER2-low ABC patients with active BMs. Further studies are needed to validate these results in larger cohorts. This trial is registered with ClinicalTrials.gov, NCT04420598.
Keywords: HER2-low; T-DXd; active brain metastases; advanced breast cancer; trastuzumab deruxtecan.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Wolff A.C., Somerfield M.R., Dowsett M., et al. Human epidermal growth factor receptor 2 testing in breast cancer: ASCO-College of American Pathologists Guideline Update. J Clin Oncol. 2023;41(22) - PubMed
-
- Tarantino P., Curigliano G., Tolaney S.M. Navigating the HER2-low paradigm in breast oncology: new standards, future horizons. Cancer Discov. 2022;12(9):2026–2030. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

