Efficacy and safety of the combination of encorafenib/cetuximab with or without binimetinib in patients with BRAF V600E-mutated metastatic colorectal cancer: an AGEO real-world multicenter study
- PMID: 39255538
- PMCID: PMC11415680
- DOI: 10.1016/j.esmoop.2024.103696
Efficacy and safety of the combination of encorafenib/cetuximab with or without binimetinib in patients with BRAF V600E-mutated metastatic colorectal cancer: an AGEO real-world multicenter study
Abstract
Background: The combination of encorafenib with cetuximab has become the standard of care in patients with BRAF V600E-mutated metastatic colorectal cancer (mCRC) after a prior systemic therapy. This study aims to describe the efficacy and safety of encorafenib/cetuximab +/- binimetinib in patients with BRAF V600E-mutated mCRC in a real-world setting.
Patients and methods: This retrospective study included patients with BRAF V600E-mutated mCRC who received this combination from January 2020 to June 2022 in 30 centers.
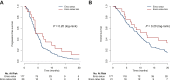
Results: A total of 201 patients were included, with 55% of women, a median age of 62 years, and an Eastern Cooperative Oncology Group performance status (ECOG-PS) >1 in 20% of cases. The main tumor characteristics were 60% of right-sided primary tumor, 11% of microsatellite instability/mismatch repair deficient phenotype, and liver and peritoneum being the two main metastatic sites (57% and 51%). Encorafenib/cetuximab +/- binimetinib was prescribed in the first, second, third, and beyond third line in 4%, 56%, 29%, and 11%, respectively, of cases, with the encorafenib/cetuximab/binimetinib combination for 21 patients (10%). With encorafenib/cetuximab treatment, 21% of patients experienced grade ≥3 adverse events (AEs), with each type of grade ≥3 AE observed in <5% of patients. The objective response rate was 32.2% and the disease control rate (DCR) was 71.2%. The median progression-free survival (PFS) was 4.5 months [95% confidence interval (CI) 3.9-5.4 months] and the median overall survival (OS) was 9.2 months (95% CI 7.8-10.8 months). In multivariable analysis, factors associated with a shorter PFS were synchronous metastases [hazard ratio (HR) 1.66, P = 0.04] and ECOG-PS >1 (HR 1.88, P = 0.007), and those associated with a shorter OS were the same factors (HR 1.71, P = 0.03 and HR 2.36, P < 0.001, respectively) in addition to treatment beyond the second line (HR 1.74, P = 0.003) and high carcinoembryonic antigen level (HR 1.72, P = 0.003).
Conclusion: This real-world study showed that in patients with BRAF V600E-mutated mCRC treated with encorafenib/cetuximab +/- binimetinib, efficacy and safety data confirm those reported in the BEACON registration trial. The main poor prognostic factors for this treatment are synchronous metastases and ECOG-PS >1.
Keywords: BRAF V600E mutation; colorectal cancer; encorafenib; real-world study; targeted therapy.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Delord J.-P., Robert C., Nyakas M., et al. Phase I dose-escalation and -expansion study of the BRAF inhibitor encorafenib (LGX818) in metastatic BRAF-mutant melanoma. Clin Cancer Res. 2017;23(18):5339–5348. - PubMed
-
- Dummer R., Ascierto P.A., Gogas H.J., et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19(5):603–615. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

