Oxidative stress promotes oral carcinogenesis via Thbs1-mediated M1-like tumor-associated macrophages polarization
- PMID: 39255693
- PMCID: PMC11414564
- DOI: 10.1016/j.redox.2024.103335
Oxidative stress promotes oral carcinogenesis via Thbs1-mediated M1-like tumor-associated macrophages polarization
Abstract
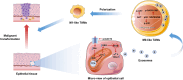
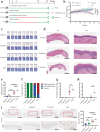
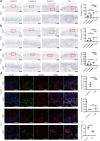
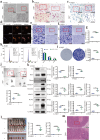
Although oxidative stress is closely associated with tumor invasion and metastasis, its' exact role and mechanism in the initial stage of oral cancer remain ambiguous. Glutamine uptake mediated by alanine-serine-cysteine transporter 2 (ASCT2) participates in glutathione synthesis to resolve oxidative stress. Currently, we firstly found that ASCT2 deletion caused oxidative stress in oral mucosa and promoted oral carcinogenesis induced by 4-Nitroquinoline-1-oxide (4-NQO) using transgenic mice of ASCT2 knockout in oral epithelium. Subsequently, we identified an upregulated gene Thbs1 linked to macrophage infiltration by mRNA sequencing and immunohistochemistry. Importantly, multiplex immunohistochemistry showed M1-like tumor-associated macrophages (TAMs) were enriched in cancerous area. Mechanically, targeted ASCT2 effectively curbed glutamine uptake and caused intracellular reactive oxygen species (ROS) accumulation, which upregulated Thbs1 in oral keratinocytes and then activated p38, Akt and SAPK/JNK signaling to polarize M1-like TAMs via exosome-transferred pathway. Moreover, we demonstrated M1-like TAMs promoted malignant progression of oral squamous cell carcinoma (OSCC) both in vitro and in vivo by a DOK transformed cell line induced by 4-NQO. All these results establish that oxidative stress triggered by ASCT2 deletion promotes oral carcinogenesis through Thbs1-mediated M1 polarization, and indicate that restore redox homeostasis is a new approach to prevent malignant progression of oral potentially malignant disorders.
Keywords: ASCT2; Macrophages; Oral carcinogenesis; ROS; Thbs1.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Xiaoan Tao reports was provided by National Natural Science Foundation of China. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

