Fully defined NGN2 neuron protocol reveals diverse signatures of neuronal maturation
- PMID: 39255791
- PMCID: PMC11440061
- DOI: 10.1016/j.crmeth.2024.100858
Fully defined NGN2 neuron protocol reveals diverse signatures of neuronal maturation
Abstract
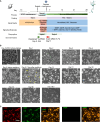
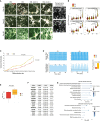
NGN2-driven induced pluripotent stem cell (iPSC)-to-neuron conversion is a popular method for human neurological disease modeling. In this study, we present a standardized approach for generating neurons utilizing clonal, targeted-engineered iPSC lines with defined reagents. We demonstrate consistent production of excitatory neurons at scale and long-term maintenance for at least 150 days. Temporal omics, electrophysiological, and morphological profiling indicate continued maturation to postnatal-like neurons. Quantitative characterizations through transcriptomic, imaging, and functional assays reveal coordinated actions of multiple pathways that drive neuronal maturation. We also show the expression of disease-related genes in these neurons to demonstrate the relevance of our protocol for modeling neurological disorders. Finally, we demonstrate efficient generation of NGN2-integrated iPSC lines. These workflows, profiling data, and functional characterizations enable the development of reproducible human in vitro models of neurological disorders.
Keywords: CP: Neuroscience; CP: Stem cell; NGN2; disease modeling; iPSC; multi-omics profiling; neuron maturation; neuronal differentiation.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests All authors are employees of Genentech, Inc., a member of the Roche group.
Figures






References
-
- Burke E.E., Chenoweth J.G., Shin J.H., Collado-Torres L., Kim S.-K., Micali N., Wang Y., Colantuoni C., Straub R.E., Hoeppner D.J., et al. Dissecting transcriptomic signatures of neuronal differentiation and maturation using iPSCs. Nat. Commun. 2020;11:462. doi: 10.1038/s41467-019-14266-z. - DOI - PMC - PubMed
-
- van de Leemput J., Boles N.C., Kiehl T.R., Corneo B., Lederman P., Menon V., Lee C., Martinez R.A., Levi B.P., Thompson C.L., et al. CORTECON: a temporal transcriptome analysis of in vitro human cerebral cortex development from human embryonic stem cells. Neuron. 2014;83:51–68. doi: 10.1016/j.neuron.2014.05.013. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

