Scission-Enhanced Molecular Imaging (SEMI)
- PMID: 39255972
- PMCID: PMC11488501
- DOI: 10.1021/acs.bioconjchem.4c00337
Scission-Enhanced Molecular Imaging (SEMI)
Abstract
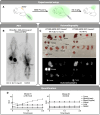
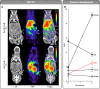
Positron emission tomography (PET) imaging methods have advanced our understanding of human biology, while targeted radiotherapeutic drug treatments are now routinely used clinically. The field is expected to grow considerably based on an expanding repertoire of available affinity ligands, radionuclides, conjugation chemistries, and their FDA approvals. With this increasing use, strategies for dose reduction have become of high interest to protect patients from unnecessary and off-target toxicity. Here, we describe a simple and powerful method, scission-enhanced molecular imaging (SEMI). The technique allows for rapid corporeal elimination of radionuclides once imaging or theranostic treatment is completed and relies on "click-to-release" bioorthogonal linkers.
Conflict of interest statement
The authors declare the following competing financial interest(s): RW has consulted for Boston Scientific, Earli, and Accure Health, none of whom contributed to or were involved in this research. MAM has received research support from Pfizer, Genentech/Roche, and Ionis Pharmaceuticals. TSCN has received research support from Lanthus and Bayer. None of these activities relate to the manuscript.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

