Cathepsin C (dipeptidyl peptidase 1) inhibition in adults with bronchiectasis: AIRLEAF, a phase II randomised, double-blind, placebo-controlled, dose-finding study
- PMID: 39255990
- PMCID: PMC11694546
- DOI: 10.1183/13993003.01551-2024
Cathepsin C (dipeptidyl peptidase 1) inhibition in adults with bronchiectasis: AIRLEAF, a phase II randomised, double-blind, placebo-controlled, dose-finding study
Abstract
Background: Bronchiectasis is characterised by uncontrolled neutrophil serine protease (NSP) activity. Cathepsin C (CatC; dipeptidyl peptidase 1) activates NSPs during neutrophil maturation. CatC inhibitors can potentially reduce neutrophil-mediated lung damage. This phase II, randomised, double-blind, placebo-controlled trial (AIRLEAF®; clinicaltrials.gov identifier NCT05238675) evaluated efficacy, safety and optimal dosing of BI 1291583, a novel, reversible CatC inhibitor, in adults with bronchiectasis.
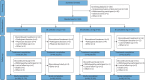
Methods: In total, 322 participants were randomised (2:1:1:2) to receive one of three oral doses of BI 1291583 (1 mg/2.5 mg/5 mg) or placebo for 24-48 weeks. A multiple comparison procedure and modelling approach was used to demonstrate a nonflat dose-response curve based on the time to first pulmonary exacerbation up to week 48. In addition, efficacy of individual BI 1291583 doses was evaluated based on the frequency of exacerbations, severe exacerbations (fatal or leading to hospitalisation and/or intravenous antibiotic administration), lung function and quality of life.
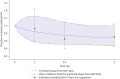
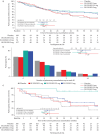
Results: A significant dose-dependent benefit of BI 1291583 over placebo was established based on time to first exacerbation (shape: maximum effect curve 1; adjusted p=0.0448). Treatment with BI 1291583 5 mg and 2.5 mg numerically reduced the risk of an exacerbation compared with placebo (hazard ratio (95% CI) 0.71 (0.48 to 1.05) and 0.66 (0.40 to 1.08), respectively; both p>0.05). BI 1291583 2.5 mg showed numerically better efficacy compared with 5 mg across several end-points; 1 mg was similar to placebo. The safety profile of BI 1291583 was similar to placebo.
Conclusion: Treatment with BI 1291583 resulted in a reduction in the risk of experiencing an exacerbation in adults with bronchiectasis.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: J.D. Chalmers reports support for the present manuscript from Boehringer Ingelheim, grants or contracts from AstraZeneca, Boehringer Ingelheim, Genentech, Gilead Sciences, GlaxoSmithKline, Grifols, Insmed, Novartis and Trudell Medical Group, consultancy fees from Antabio, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Grifols, Insmed, Janssen, Novartis, Pfizer, Trudell Medical Group and Zambon, and is the current Chief Editor of the European Respiratory Journal. M. Shteinberg reports support for the present manuscript from Boehringer Ingelheim, grants or contracts from GlaxoSmithKline, Insmed, Novartis, Tel Aviv League for Lung Diseases and Trudell Medical Group, consultancy fees from AstraZeneca, Boehringer Ingelheim, Dexcel, GlaxoSmithKline, Kamada, Synchrony Medical, Trumed, Vertex Pharmaceuticals and Zambon, payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Insmed, Kamada, Novartis, Physioassist, Sanofi and Teva, participation on a data safety monitoring board or advisory board for AstraZeneca, Boehringer Ingelheim and Bonus Biotherapeutics, support for attending meetings and/or travel from Actelion, AstraZeneca Israel, Boehringer Ingelheim Israel, GlaxoSmithKline Israel, Kamada, Novartis and Rafa, leadership or fiduciary roles as AJRCCM associate editor, management board member of the Israeli Pulmonology Society, Israeli Society for Tuberculosis and Mycobacterial Diseases and EMBARC, editorial board member of ERJ and Chest, and taskforce member for ERS bronchiectasis guidelines, and receipt of oPEP devices for clinical trial from Trudell Medical Group. M.A. Mall reports support for the present manuscript from Boehringer Ingelheim, grants or contracts from Boehringer Ingelheim, German Innovation Fund, German Ministry for Education and Research (BMBF), German Research Foundation (DFG) and an independent medical grant from Vertex Pharmaceuticals with payments made to the institution, consultancy fees from AbbVie, Antabio, Arrowhead, Boehringer Ingelheim, Enterprise Therapeutics, Kither Biotech, Pieris Pharmaceuticals, Recode, Santhera, Splisense and Vertex Pharmaceuticals, payment or honoraria for lectures from Vertex Pharmaceuticals, travel reimbursement received for participation in advisory board meetings for Boehringer Ingelheim and Vertex Pharmaceuticals, and fees for participation on an advisory board from AbbVie, Boehringer Ingelheim, Enterprise Therapeutics, Kither Biotech, Pari and Vertex Pharmaceuticals. M.A. Mall also reports that he is inventor on an issued patent filed by the University of North Carolina at Chapel Hill, describing the Scnn1b-transgenic mouse. M.A. Mall is an unpaid fellow of ERS. A.E. O'Donnell reports support for the present manuscript from Boehringer Ingelheim, grants or contracts from AN2, Armata, Boehringer Ingelheim, Insmed, Paratek, Renovian, Spero and Zambon, and consultancy fees from Boehringer Ingelheim and Insmed, payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from Academic CME, New York University School of Medicine, Peer View Institute and Vinidico, and a leadership or fiduciary role on the US Bronchiectasis and NTM Research Registry steering committee. H. Watz reports support for the present manuscript from Boehringer Ingelheim, and consultancy fees, payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events, payment for expert testimony, support for attending meetings and/or travel, and participation on a data safety monitoring board or advisory board from AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Novartis and Sanofi. H. Watz is a chair for the COPD guideline group, German Respiratory Society and scientific coordinator for COPD at the German Center for Lung Research (DZL). A. Gupta is an employee of Boehringer Ingelheim. E. Frahm is an employee of Boehringer Ingelheim. A. Eleftheraki is an employee of Boehringer Ingelheim. J. Rauch is an employee of Boehringer Ingelheim. S.H. Chotirmall reports support for the present manuscript from Boehringer Ingelheim, grants or contracts from Singapore Ministry of Education under its AcRF Tier 1 Grant (RT1/22), Singapore Ministry of Health's National Medical Research Council under its Clinician Scientist Award (MOH-000710), Singapore Ministry of Health's National Medical Research Council under its Clinician Scientist-Individual Research Grant (MOH-001356) and Singapore Ministry of Health's National Medical Research Council Open Fund Individual Research Grant (MOH-000955) with payments made to the institution, consultancy fees from Boehringer Ingelheim, CSL Behring and Pneumagen Ltd, lecture fees from AstraZeneca and Chiesi Farmaceutici, and participation on a data safety monitoring board or advisory board for Inovio Pharmaceuticals Inc. and Imam Abdulrahman Bin Faisal University. A.W. Armstrong reports grants or contracts from AbbVie, ASLAN, BMS, Dermavant Sciences, Dermira, Eli Lilly, Galderma, Incyte, Janssen, LEO Pharma, Meiji Seika Pharma Co., Modernizing Medicine, Nimbus Therapeautics, Novartis, Ortho Dermatologics, Pfizer, Sanofi Genzyme, UCB and Ventyx Biosciences, consulting fees from AbbVie, ASLAN, Almirall, Amgen, Arcutis, Beiersdorf, BMS, Dermavant, EPI Health, Eli Lilly, Janssen, LEO Pharma, Mindera, Nimbus, Organon & Co., Sanofi, Sun Pharma, Takeda and Ventyx Biosciences, payment or honoraria for lectures, presentations, manuscript writing or educational events from AbbVie, Amgen, BMS, Galderma, Janssen, Mindera, Organon & Co., Sanofi and Takeda, participation on a data safety monitoring board or advisory board for Incyte, Regeneron, UCB, Boehringer Ingelheim and Parexel, and leadership or fiduciary role as Board of Director Elect, American Academy of Dermatology. P. Eickholz reports support for the present manuscript and consulting fees from Boehringer Ingelheim, and participation on a data safety monitoring board or advisory board for Boehringer Ingelheim. N. Hasegawa reports support for the present manuscript and royalties or licences from Boehringer Ingelheim, and patents planned, issued or pending for Boehringer Ingelheim, grants or contracts from Insmed, consultancy fees from Boehringer Ingelheim and Insmed, and payment or honoraria for lectures from Insmed. W. Sauter is an employee of Boehringer Ingelheim. P.J. McShane reports support for the present manuscript and consultancy fees from Boehringer Ingelheim, payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from Insmed, participation on a data safety monitoring board or advisory board with Boehringer Ingelheim (AIRLEAF trial), Insmed (Aspen trial) and Spero, and the following financial or non-financial interests: principal investigator for AN2 Therapeutics (Epetraborole), Armata, Boehringer Ingelheim (AIRLEAF), Electromed, Insmed (Aspen trial), MannKind, Paratek (oral omadacycline), Renovian (ARINA-1) and Spero.
Figures

Comment in
-
Disarming the cavalry: targeting neutrophils to limit collateral damage in non-CF bronchiectasis.Eur Respir J. 2025 Jan 2;65(1):2401804. doi: 10.1183/13993003.01804-2024. Print 2025 Jan. Eur Respir J. 2025. PMID: 39746771 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
