The dual nature of DNA damage response in obesity and bariatric surgery-induced weight loss
- PMID: 39256343
- PMCID: PMC11387396
- DOI: 10.1038/s41419-024-06922-0
The dual nature of DNA damage response in obesity and bariatric surgery-induced weight loss
Abstract
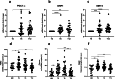
This novel study applies targeted functional proteomics to examine tissues and cells obtained from a cohort of individuals with severe obesity who underwent bariatric surgery (BS), using a Reverse-Phase Protein Array (RPPA). In obese individuals, visceral adipose tissue (VAT), but not subcutaneous adipose tissue (SAT), shows activation of DNA damage response (DDR) markers including ATM, ATR, histone H2AX, KAP1, Chk1, and Chk2, alongside senescence markers p16 and p21. Additionally, stress-responsive metabolic markers, such as survivin, mTOR, and PFKFB3, are specifically elevated in VAT, suggesting both cellular stress and metabolic dysregulation. Conversely, peripheral blood mononuclear cells (PBMCs), while exhibiting elevated mTOR and JNK levels, did not present significant changes in DDR or senescence markers. Following BS, unexpected increases in phosphorylated ATM, ATR, and KAP1 levels, but not in Chk1 and Chk2 nor in senescence markers, were observed. This was accompanied by heightened levels of survivin and mTOR, along with improvement in markers of mitochondrial quality and health. This suggests that, following BS, pro-survival pathways involved in cellular adaptation to various stressors and metabolic alterations are activated in circulating PBMCs. Moreover, our findings demonstrate that the DDR has a dual nature. In the case of VAT from individuals with obesity, chronic DDR proves to be harmful, as it is associated with senescence and chronic inflammation. Conversely, after BS, the activation of DDR proteins in PBMCs is associated with a beneficial survival response. This response is characterized by metabolic redesign and improved mitochondrial biogenesis and functionality. This study reveals physiological changes associated with obesity and BS that may aid theragnostic approaches.
© 2024. The Author(s).
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Figures





References
-
- Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. - PubMed
-
- Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80. - PubMed
-
- Interventions. IWGotEoC-P. Absence of excess body fatness. Absence of excess body fatness. IARC Handbooks of Cancer Prevention. Lyon (FR) 2021.
MeSH terms
Grants and funding
- 2013-02357791/Ministero della Salute (Ministry of Health, Italy)
- 2013-02357791/Ministero della Salute (Ministry of Health, Italy)
- E.F.2018/Istituto Superiore di Sanità (ISS)
- E.F.2018/Istituto Superiore di Sanità (ISS)
- 2017L8Z2EM/Ministero dell'Istruzione, dell'Università e della Ricerca (Ministry of Education, University and Research)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

