Dialkylation of CF2 unit enabled by cobalt electron-shuttle catalysis
- PMID: 39256384
- PMCID: PMC11387730
- DOI: 10.1038/s41467-024-51532-1
Dialkylation of CF2 unit enabled by cobalt electron-shuttle catalysis
Abstract
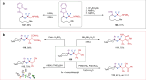
The incorporation of difluoromethylene (CF2) group into chemical molecules often imparts desirable properties such as lipophilicity, binding affinity, and thermal stability. Consequently, the increasing demand for gem-difluoroalkylated compounds in drug discovery and materials science has continued to drive the development of practical methods for their synthesis. However, traditional synthetic methods such as deoxofluorination often confront challenges including complicated substrate synthesis sequences and poor functional group compatibility. In this context, we herein report a metal electron-shuttle catalyzed, modular synthetic methodology for difluoroalkylated compounds by assembling two C(sp3) fragments across CF2 unit in a single step. The approach harnesses a difluoromethylene synthon as a biradical linchpin, achieving the construction of two C(sp3)-CF2 bonds through radical addition to two different π-unsaturated molecules. This catalytic protocol is compatible with broad range of coupling partners including diverse olefins, iminiums, and hydrazones, supporting endeavors in the efficient construction of C(sp3)-rich difluoroalkylated molecules.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Gribble, G. W. A recent survey of naturally occurring organohalogen compounds. Environ. Chem.12, 396–405 (2015). 10.1071/EN15002 - DOI
-
- Mimura, H., Kawada, K., Yamashita, T., Sakamoto, T. & Kikugawa, Y. Trifluoroacetaldehyde: a useful industrial bulk material for the synthesis of trifluoromethylated amino compounds. J. Fluor. Chem.131, 477–486 (2010). 10.1016/j.jfluchem.2009.12.023 - DOI
-
- Blackburn, G. M. & Kent, D. E. Monofluoro- and difluoromethylenebisphosphonic acids: isopolar analogues of pyrophosphoric acid. J. Chem. Soc. Chem. Commun.1981, 930–932 (1981). 10.1039/c39810000930 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

