A mouse protozoan boosts antigen-specific mucosal IgA responses in a specific lipid metabolism- and signaling-dependent manner
- PMID: 39256385
- PMCID: PMC11387640
- DOI: 10.1038/s41467-024-52336-z
A mouse protozoan boosts antigen-specific mucosal IgA responses in a specific lipid metabolism- and signaling-dependent manner
Abstract
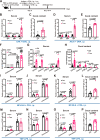
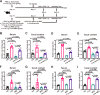
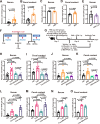
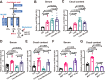
IgA antibodies play an important role in mucosal immunity. However, there is still no effective way to consistently boost mucosal IgA responses, and the factors influencing these responses are not fully understood. We observed that colonization with the murine intestinal symbiotic protozoan Tritrichomonas musculis (T.mu) boosted antigen-specific mucosal IgA responses in wild-type C57BL/6 mice. This enhancement was attributed to the accumulation of free arachidonic acid (ARA) in the intestinal lumen, which served as a signal to stimulate the production of antigen-specific mucosal IgA. When ARA was prevented from undergoing its downstream metabolic transformation using the 5-lipoxygenase inhibitor zileuton or by blocking its downstream biological signaling through genetic deletion of the Leukotriene B4 receptor 1 (Blt1), the T.mu-mediated enhancement of antigen-specific mucosal IgA production was suppressed. Moreover, both T.mu transfer and dietary supplementation of ARA augmented the efficacy of an oral vaccine against Salmonella infection, with this effect being dependent on Blt1. Our findings elucidate a tripartite circuit linking nutrients from the diet or intestinal microbiota, host lipid metabolism, and the mucosal humoral immune response.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Western diet reduces small intestinal intraepithelial lymphocytes via FXR-Interferon pathway.Mucosal Immunol. 2024 Oct;17(5):1019-1028. doi: 10.1016/j.mucimm.2024.07.001. Epub 2024 Jul 9. Mucosal Immunol. 2024. PMID: 38992433 Free PMC article.
-
Diet-derived LPS determines intestinal IgA induction and repertoire characteristics independently of the microbiota.Immunity. 2025 Jul 8;58(7):1778-1793.e7. doi: 10.1016/j.immuni.2025.05.024. Epub 2025 Jun 26. Immunity. 2025. PMID: 40578363
-
Role of mucosal IgA antibodies as novel therapies to enhance mucosal barriers.Semin Immunopathol. 2024 Nov 20;47(1):1. doi: 10.1007/s00281-024-01027-4. Semin Immunopathol. 2024. PMID: 39567378 Free PMC article. Review.
-
Molecular feature-based classification of retroperitoneal liposarcoma: a prospective cohort study.Elife. 2025 May 23;14:RP100887. doi: 10.7554/eLife.100887. Elife. 2025. PMID: 40407808 Free PMC article.
-
All roads lead to IgA: Mapping the many pathways of IgA induction in the gut.Immunol Rev. 2024 Sep;326(1):66-82. doi: 10.1111/imr.13369. Epub 2024 Jul 24. Immunol Rev. 2024. PMID: 39046160 Review.
Cited by
-
Dietary Nitrogen and Its Role in the Gut Microbiome and Inflammatory Bowel Disease: A Narrative Review.Nutrients. 2025 Jul 20;17(14):2373. doi: 10.3390/nu17142373. Nutrients. 2025. PMID: 40732998 Free PMC article. Review.
-
Colonization resistance: the role of gut microbiota in preventing Salmonella invasion and infection.Gut Microbes. 2024 Jan-Dec;16(1):2424914. doi: 10.1080/19490976.2024.2424914. Epub 2024 Nov 8. Gut Microbes. 2024. PMID: 39514544 Free PMC article. Review.
References
-
- Liew, F. Y., Russell, S. M., Appleyard, G., Brand, C. M. & Beale, J. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T cell reactivity. Eur. J. Immunol.14, 350–356 (1984). 10.1002/eji.1830140414 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

