WHO International Standards for antibodies to HPV6 HPV11 HPV31 HPV33 HPV45 HPV52 and HPV58
- PMID: 39256440
- PMCID: PMC11387505
- DOI: 10.1038/s41541-024-00949-2
WHO International Standards for antibodies to HPV6 HPV11 HPV31 HPV33 HPV45 HPV52 and HPV58
Abstract
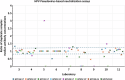
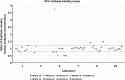
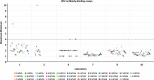
Previously established World Health Organization (WHO) International Standards (IS) for anti-HPV16 and HPV18 antibodies are used to harmonize results across human papillomavirus (HPV) serology assays. Here, we present an international collaborative study to establish ISs for antibodies against HPV6 (NIBSC code 19/298), HPV11 (20/174), HPV31 (20/176), HPV33 (19/290), HPV45 (20/178), HPV52 (19/296) and HPV58 (19/300). The candidate standards were prepared using sera from naturally infected individuals. Each candidate was shown to be monospecific for reactivity against its indicated HPV type except for the HPV11 candidate, which was also reactive against other types. Expression of antibody levels relative to the relevant candidate IS reduced inter-laboratory variation allowing greater comparability between laboratories. Based on these results, the WHO Expert Committee on Biological Standardization established each of the 7 candidates as the 1st IS for antiserum to its indicated HPV type for use in the standardization of HPV pseudovirion-based neutralization and antibody-binding assays.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- WHO. Global strategy to accelerate the elimination of cervical cancer as a public health problem. (World Health Organization, Geneva, 2020).
-
- WHO. World Health Assembly adopts global strategy to accelerate cervical cancer elimination, https://www.who.int/news/item/19-08-2020-world-health-assembly-adopts-gl... (2020).
-
- WHO. Recommendations to assure the quality, safety and efficacy of recombinant human papillomavirus virus-like particle vaccines in WHO Expert Committee on Biological Standardization, Sixty-sixth report, Annex 4, WHO Technical Report Series No. 999. (World Health Organization, Geneva, 2016).
-
- IARC HPV Working Group. Primary End-points for Prophylactic HPV Vaccine Trials. (International Agency for Research on Cancer, Lyon, FR, 2014). https://www.ncbi.nlm.nih.gov/books/NBK304971/. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

