The global phosphorylation landscape of mouse oocytes during meiotic maturation
- PMID: 39256562
- PMCID: PMC11480333
- DOI: 10.1038/s44318-024-00222-1
The global phosphorylation landscape of mouse oocytes during meiotic maturation
Abstract
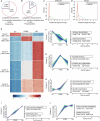
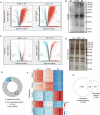
Phosphorylation is a key post-translational modification regulating protein function and biological outcomes. However, the phosphorylation dynamics orchestrating mammalian oocyte development remains poorly understood. In the present study, we apply high-resolution mass spectrometry-based phosphoproteomics to obtain the first global in vivo quantification of mouse oocyte phosphorylation. Of more than 8000 phosphosites, 75% significantly oscillate and 64% exhibit marked upregulation during meiotic maturation, indicative of the dominant regulatory role. Moreover, we identify numerous novel phosphosites on oocyte proteins and a few highly conserved phosphosites in oocytes from different species. Through functional perturbations, we demonstrate that phosphorylation status of specific sites participates in modulating critical events including metabolism, translation, and RNA processing during meiosis. Finally, we combine inhibitor screening and enzyme-substrate network prediction to discover previously unexplored kinases and phosphatases that are essential for oocyte maturation. In sum, our data define landscape of the oocyte phosphoproteome, enabling in-depth mechanistic insights into developmental control of germ cells.
Keywords: Kinase; Meiosis; Oocyte; Phosphatase; Phosphorylation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures










References
-
- Adhikari D, Flohr G, Gorre N, Shen Y, Yang H, Lundin E, Lan Z, Gambello MJ, Liu K (2009) Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol Hum Reprod 15:765–770 - PubMed
-
- Adhikari D, Liu K (2014) The regulation of maturation promoting factor during prophase I arrest and meiotic entry in mammalian oocytes. Mol Cell Endocrinol 382:480–487 - PubMed
-
- Adhikari D, Zheng W, Shen Y, Gorre N, Ning Y, Halet G, Kaldis P, Liu K (2012) Cdk1, but not Cdk2, is the sole Cdk that is essential and sufficient to drive resumption of meiosis in mouse oocytes. Hum Mol Genet 21:2476–2484 - PubMed
MeSH terms
Substances
Grants and funding
- 81925014/MOST | National Natural Science Foundation of China (NSFC)
- 82221005/MOST | National Natural Science Foundation of China (NSFC)
- 82101736/MOST | National Natural Science Foundation of China (NSFC)
- 2021YFC2700400/MOST | National Key Research and Development Program of China (NKPs)
- BK20190615/JST | Natural Science Foundation of Jiangsu Province (Jiangsu Natural Science Foundation)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

