Dihydroartemisinin Regulated the MMP-Mediated Cellular Microenvironment to Alleviate Rheumatoid Arthritis
- PMID: 39257420
- PMCID: PMC11385568
- DOI: 10.34133/research.0459
Dihydroartemisinin Regulated the MMP-Mediated Cellular Microenvironment to Alleviate Rheumatoid Arthritis
Abstract
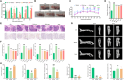
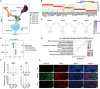
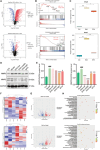
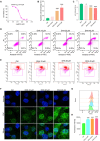
Rheumatoid arthritis (RA) is an autoimmune disease with features of synovial inflammation, cartilage erosion, bone destruction, and pain and is currently lacking a satisfactory treatment strategy. Dihydroartemisinin (DHA), the active metabolite of artemisinin, has exhibited outstanding suppressive effects on RA without obvious side effects. However, the underlying mechanisms remain unclear, which limits its further clinical application. The purpose of this study is to reveal the pharmacodynamic mechanism of DHA against RA by means of a combination of single-cell RNA sequencing (RNA-seq), proteomics, as well as transcriptomics both in vivo and in vitro. In our results, DHA effectively reduced the degree of redness, swelling, and pain in RA rats and dramatically changed the synovial tissue microenvironment under the pathological state. Within this microenvironment, fibroblasts, macrophages, B cells, and endothelial cells were the major affected cell types, primarily through DHA targeting the extracellular matrix (ECM) structural constituent signaling pathway. In addition, we confirmed that DHA regulated the ECM by modulating matrix metalloproteinase 2 (MMP2) and MMP3 in the synovial tissue of RA rats. Moreover, DHA induced apoptosis in MH7A cells, further validating the bioinformatics data. In conclusion, DHA effectively reduced the inflammatory response and improved the immune microenvironment in synovial tissue by inhibiting MMP2 and MMP3. Our findings provide a basis for the application of DHA in the treatment of RA.
Copyright © 2024 Qiuyan Guo et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures


References
-
- Zhang C, Ma P, Qin A, Wang L, Dai K, Liu Y, Zhao J, Lu Z. Current immunotherapy strategies for rheumatoid arthritis: The immunoengineering and delivery systems. Research. 2023;6:0220.
-
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. - PubMed
-
- Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, McInnes IB, Sepriano A, Vollenhoven RF, Wit M, et al. . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699. - PubMed
-
- Du Y, Li C, Zhang Y, Xiong W, Wang F, Wang J, Zhang Y, Deng L, Li X, Chen W, et al. . In situ-activated phospholipid-mimic artemisinin prodrug via injectable hydrogel Nano/microsphere for rheumatoid arthritis therapy. Research. 2022;2022:0003.
-
- Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJ, Kvien TK, Dougados M, Radner H, Atzeni F, et al. . EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76(1):17–28. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

