Different polarization and functionality of CD4+ T helper subsets in people with post-COVID condition
- PMID: 39257580
- PMCID: PMC11385313
- DOI: 10.3389/fimmu.2024.1431411
Different polarization and functionality of CD4+ T helper subsets in people with post-COVID condition
Abstract
Introduction: After mild COVID-19 that does not require hospitalization, some individuals develop persistent symptoms that may worsen over time, producing a multisystemic condition termed Post-COVID condition (PCC). Among other disorders, PCC is characterized by persistent changes in the immune system that may not be solved several months after COVID-19 diagnosis.
Methods: People with PCC were recruited to determine the distribution and functionality of CD4+ T helper (Th) subsets in comparison with individuals with mild, severe, and critical presentations of acute COVID-19 to evaluate their contribution as risk or protective factors for PCC.
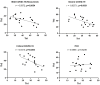
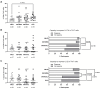
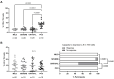
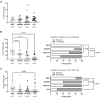
Results: People with PCC showed low levels of Th1 cells, similar to individuals with severe and critical COVID-19, although these cells presented a higher capacity to express IFNγ in response to stimulation. Th2/Th1 correlation was negative in individuals with acute forms of COVID-19, but there was no significant Th2/Th1 correlation in people with PCC. Th2 cells from people with PCC presented high capacity to express IL-4 and IL-13, which are related to low ventilation and death associated with COVID-19. Levels of proinflammatory Th9 and Th17 subsets were significantly higher in people with PCC in comparison with acute COVID-19, being Th1/Th9 correlation negative in these individuals, which probably contributed to a more pro-inflammatory than antiviral scenario. Th17 cells from approximately 50% of individuals with PCC had no capacity to express IL-17A and IL-22, similar to individuals with critical COVID-19, which would prevent clearing extracellular pathogens. Th2/Th17 correlation was positive in people with PCC, which in the absence of negative Th1/Th2 correlation could also contribute to the proinflammatory state. Finally, Th22 cells from most individuals with PCC had no capacity to express IL-13 or IL-22, which could increase tendency to reinfections due to impaired epithelial regeneration.
Discussion: People with PCC showed skewed polarization of CD4+ Th subsets with altered functionality that was more similar to individuals with severe and critical presentations of acute COVID-19 than to people who fully recovered from mild disease. New strategies aimed at reprogramming the immune response and redirecting CD4+ Th cell polarization may be necessary to reduce the proinflammatory environment characteristic of PCC.
Keywords: CD4+ T cells; T helper polarization; Th1; Th17; Th2; cytokines; post-covid condition.
Copyright © 2024 Sánchez-Menéndez, de la Calle-Jiménez, Mateos, Vigón, Fuertes, Murciano Antón, San José, García-Gutiérrez, Cervero, Torres and Coiras.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Clinical management of COVID-19: Living guideline, 18 August 2023 . Available online at: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-clinical-... (Accessed May, 2024).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

