This is a preprint.
Limosilactobacillus reuteri promotes the expression and secretion of enteroendocrine- and enterocyte-derived hormones
- PMID: 39257733
- PMCID: PMC11384013
- DOI: 10.1101/2024.08.30.610555
Limosilactobacillus reuteri promotes the expression and secretion of enteroendocrine- and enterocyte-derived hormones
Update in
-
Limosilactobacillus reuteri promotes the expression and secretion of enteroendocrine- and enterocyte-derived hormones.FASEB J. 2025 Mar 31;39(6):e70408. doi: 10.1096/fj.202401669R. FASEB J. 2025. PMID: 40098558 Free PMC article.
Abstract
Observations that intestinal microbes can beneficially impact host physiology have prompted investigations into the therapeutic usage of such microbes in a range of diseases. For example, the human intestinal microbe Limosilactobacillus reuteri strains ATCC PTA 6475 and DSM 17938 are being considered for use for intestinal ailments including colic, infection, and inflammation as well as non-intestinal ailments including osteoporosis, wound healing, and autism spectrum disorder. While many of their beneficial properties are attributed to suppressing inflammatory responses in the gut, we postulated that L. reuteri may also regulate hormones of the gastrointestinal tract to affect physiology within and outside of the gut. To determine if L. reuteri secreted factors impact the secretion of enteric hormones, we treated an engineered jejunal organoid line, NGN3-HIO, which can be induced to be enriched in enteroendocrine cells, with L. reuteri 6475 or 17938 conditioned medium and performed transcriptomics. Our data suggest that these L. reuteri strains affect the transcription of many gut hormones, including vasopressin and luteinizing hormone subunit beta, which have not been previously recognized as being produced in the gut epithelium. Moreover, we find that these hormones appear to be produced in enterocytes, in contrast to canonical gut hormones which are produced in enteroendocrine cells. Finally, we show that L. reuteri conditioned media promotes the secretion of several enteric hormones including serotonin, GIP, PYY, vasopressin, and luteinizing hormone subunit beta. These results support L. reuteri affecting host physiology through intestinal hormone secretion, thereby expanding our understanding of the mechanistic actions of this microbe.
Keywords: GIP; Lactobacillus; PYY; adipolin; enterocyte; enteroendocrine; hormone; kisspeptin; luteinizing hormone; small intestine; vasopressin.
Conflict of interest statement
COI statement: The authors declare no conflict of interest.
Figures



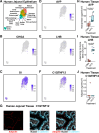
References
-
- Savino F., Pelle E., Palumeri E., Oggero R. & Miniero R. Lactobacillus reuteri (American Type Culture Collection Strain 55730) Versus Simethicone in the Treatment of Infantile Colic: A Prospective Randomized Study. Pediatrics 119, e124–e130 (2007). - PubMed
-
- Nilsson A. G., Sundh D., Bäckhed F. & Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J. Intern. Med. 284, 307–317 (2018). - PubMed
-
- Lin Y. P., Thibodeaux C. H., Peña J. A., Ferry G. D. & Versalovic J. Probiotic Lactobacillus reuteri suppress proinflammatory cytokines via c-Jun. Inflamm. Bowel Dis. 14, 1068–1083 (2008). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
