This is a preprint.
Extensive transmission and variation in a functional receptor for praziquantel resistance in endemic Schistosoma mansoni
- PMID: 39257780
- PMCID: PMC11383708
- DOI: 10.1101/2024.08.29.610291
Extensive transmission and variation in a functional receptor for praziquantel resistance in endemic Schistosoma mansoni
Abstract
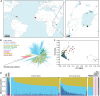
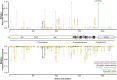
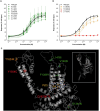
Mass-drug administration (MDA) of human populations using praziquantel monotherapy has become the primary strategy for controlling and potentially eliminating the major neglected tropical disease schistosomiasis. To understand how long-term MDA impacts schistosome populations, we analysed whole-genome sequence data of 570 Schistosoma mansoni samples (and the closely related outgroup species, S. rodhaini) from eight countries incorporating both publicly-available sequence data and new parasite material. This revealed broad-scale genetic structure across countries but with extensive transmission over hundreds of kilometres. We characterised variation across the transient receptor potential melastatin ion channel, TRPMPZQ, a target of praziquantel, which has recently been found to influence praziquantel susceptibility. Functional profiling of TRPMPZQ variants found in endemic populations identified four mutations that reduced channel sensitivity to praziquantel, indicating standing variation for resistance. Analysis of parasite infrapopulations sampled from individuals pre- and post-treatment identified instances of treatment failure, further indicative of potential praziquantel resistance. As schistosomiasis is targeted for elimination as a public health problem by 2030 in all currently endemic countries, and even interruption of transmission in selected African regions, we provide an in-depth genomic characterisation of endemic populations and an approach to identify emerging praziquantel resistance alleles.
Figures

References
-
- Control of Neglected Tropical Diseases (NTD) Guidelines Review Committee, “WHO Guideline on control and elimination of human schistosomiasis” (World Health Organization, 2022). - PubMed
-
- Lo N. C., Bezerra F. S. M., Colley D. G., Fleming F. M., Homeida M., Kabatereine N., Kabole F. M., King C. H., Mafe M. A., Midzi N., Mutapi F., Mwanga J. R., Ramzy R. M. R., Satrija F., Stothard J. R., Traoré M. S., Webster J. P., Utzinger J., Zhou X.-N., Danso-Appiah A., Eusebi P., Loker E. S., Obonyo C. O., Quansah R., Liang S., Vaillant M., Murad M. H., Hagan P., Garba A., Review of 2022 WHO guidelines on the control and elimination of schistosomiasis. Lancet Infect. Dis. 22, e327–e335 (2022). - PubMed
-
- WHO Expert Committee on the Control of Schistosomiasis, “Prevention and control of schistosomiasis and soil-transmitted helminthiasis : report of a WHO expert committee” (World Health Organ Tech Rep Ser., 2002); http://apps.who.int/iris/handle/10665/42588. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
