Silver Nanoparticles (AgNPs) Uptake by Caveolae-Dependent Endocytosis is Responsible for Their Selective Effect Towards Castration Resistant Prostate Cancer
- PMID: 39258003
- PMCID: PMC11384141
- DOI: 10.2147/IJN.S447645
Silver Nanoparticles (AgNPs) Uptake by Caveolae-Dependent Endocytosis is Responsible for Their Selective Effect Towards Castration Resistant Prostate Cancer
Abstract
Purpose: Castration Resistant Prostate Cancer (CRPC) is characterized by poor prognosis and limited therapeutic options. AgNPs functionalized with glucose (G-AgNPs) were observed cytotoxic to CRPC cell lines (PC-3 and Du-145) and not LNCaP. This study aims to evaluate AgNPs and G-AgNPs' uptake mechanisms in these cells and understand their role in the selective effect against CRPC cells.
Methods: Uptake of AgNPs and G-AgNPs was assessed through transmission electron microscopy (TEM). A microRNA (miRNAs) analysis approach was used to uncover the main molecular differences responsible for the endocytic mechanisms' regulation. Caveolin (Cav) 1 and 2 mRNA and protein levels were assessed in the three cell lines. Caveolae-dependent endocytosis was inhibited with genistein or siCav1- and siCav2- in PC-3 and Du-145 and resazurin assay was used to evaluate viability after AgNPs and G-AgNPs administration. Caveolae-dependent endocytosis was induced with Cav1+ and Cav2+ plasmids in LNCaP, resazurin assay was used to evaluate viability after AgNPs and G-AgNPs administration and TEM to assess their location.
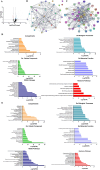
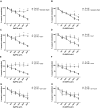
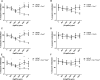
Results: AgNPs and G-AgNPs were not uptaked by LNCaP. miRNA analysis revealed 37 upregulated and 90 downregulated miRNAs. Functional enrichment analysis of miRNAs' targets resulted in enrichment of terms related to endocytosis and caveolae. We observed that Cav1 and Cav2 are not expressed in LNCaP. Inhibiting caveolae-dependent endocytosis in Du-145 and PC-3 led to a significative reduction of cytotoxic capacity of AgNPs and G-AgNPs and induction of caveolae-dependent endocytosis in LNCaP lead to a significative increase as well as their uptake by cells.
Conclusion: This study shows the potential of these AgNPs as a new therapeutic approach directed to CRPC patients, uncovers caveolae-dependent endocytosis as the uptake mechanism of these AgNPs and highlights deregulation of Cav1 and Cav2 expression as a key difference in hormone sensitive and resistant PCa cells which may be responsible for drug resistance.
Keywords: caveolins; endocytosis; prostate cancer resistant to castration; silver nanoparticles; uptake mechanism.
© 2024 Morais et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

