Effect of AMH on primordial follicle populations in mouse ovaries and human pre-pubertal ovarian xenografts during doxorubicin treatment
- PMID: 39258229
- PMCID: PMC11383774
- DOI: 10.3389/fcell.2024.1449156
Effect of AMH on primordial follicle populations in mouse ovaries and human pre-pubertal ovarian xenografts during doxorubicin treatment
Abstract
Introduction: Survival rates of the childhood cancer patients are improving, however cancer treatments such as chemotherapy may lead to infertility due to loss of the primordial follicle (PMF) reserve. Doxorubicin (DXR) is a gonadotoxic chemotherapy agent commonly used in childhood cancers. Anti-Müllerian Hormone (AMH) has been reported to have a protective effect on the mouse ovarian reserve against DXR in vivo. However, whether AMH can prevent PMF loss in conjunction with DXR in human ovarian tissue in vivo has not been determined.
Methods: In order to investigate this, we first established an optimum dose of DXR that induced PMF loss in cultured mouse ovaries and investigated the efficacy of AMH on reducing DXR-induced PMF loss in mice in vitro. Second, we investigated the effects of DXR on pre-pubertal human ovarian tissue and the ability of AMH to prevent DXR-induced damage comparing using a mouse xenograft model with different transplantation sites.
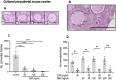
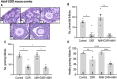
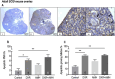
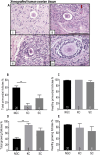
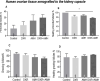
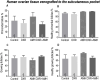
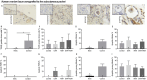
Results: Mouse ovaries treated with DXR in vitro and in vivo had reduced PMF populations and damaged follicle health. We did not observe effect of DXR-induced PMF loss or damage to follicle/stromal health in human ovarian cortex, this might have been due to an insufficient dose or duration of DXR. Although AMH does not prevent DXR-induced PMF loss in pre-pubertal and adult mouse ovaries, in mouse ovaries treated with higher concentration of AMH in vitro, DXR did not cause a significant loss in PMFs. This is the first study to illustrate an effect of AMH on DXR-induced PMF loss on pre-pubertal mouse ovaries. However, more experiments with higher doses of AMH and larger sample size are needed to confirm this finding.
Discussion: We did not observe that AMH could prevent DXR-induced PMF loss in mouse ovaries in vivo. Further studies are warranted to investigate whether AMH has a protective effect against DXR in xenotransplanted human ovarian tissue. Thus, to obtain robust evidence about the potential of AMH in fertility preservation during chemotherapy treatment, alternative AMH administration strategies need to be explored alongside DXR administration to fully interrogate the effect of DXR and AMH on human xenografted tissues.
Keywords: anti-Müllerian hormone; doxorubicin; fertility preservation; follicle; human ovarian xenograft; ovarian tissue; ovary; primordial follicles.
Copyright © 2024 Wei, Bjarkadottir, Nadjaja, Sheikh, Fatum, Lane and Williams.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Dual suppression of follicle activation pathways completely prevents the cyclophosphamide-induced loss of ovarian reserve.Hum Reprod. 2023 Jun 1;38(6):1086-1098. doi: 10.1093/humrep/dead064. Hum Reprod. 2023. PMID: 37015102
-
AMH prevents primordial ovarian follicle loss and fertility alteration in cyclophosphamide-treated mice.FASEB J. 2019 Jan;33(1):1278-1287. doi: 10.1096/fj.201801089R. Epub 2018 Aug 16. FASEB J. 2019. PMID: 30113879
-
The PARP inhibitor, olaparib, depletes the ovarian reserve in mice: implications for fertility preservation.Hum Reprod. 2020 Aug 1;35(8):1864-1874. doi: 10.1093/humrep/deaa128. Hum Reprod. 2020. PMID: 32604417
-
Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications.Hum Reprod Update. 2014 Sep-Oct;20(5):688-701. doi: 10.1093/humupd/dmu020. Epub 2014 May 12. Hum Reprod Update. 2014. PMID: 24821925 Review.
-
Translational Physiology of Anti-Müllerian Hormone: Clinical Applications in Female Fertility Preservation and Cancer Treatment.Front Endocrinol (Lausanne). 2021 Sep 7;12:689532. doi: 10.3389/fendo.2021.689532. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34557157 Free PMC article. Review.
Cited by
-
Single-Cell Transcriptomic Analysis of the Potential Mechanisms of Follicular Development in Stra8-Deficient Mice.Int J Mol Sci. 2025 Apr 15;26(8):3734. doi: 10.3390/ijms26083734. Int J Mol Sci. 2025. PMID: 40332359 Free PMC article.
References
-
- Barpe D. R., Rosa D. D., Froehlich P. E. (2010). Pharmacokinetic evaluation of doxorubicin plasma levels in normal and overweight patients with breast cancer and simulation of dose adjustment by different indexes of body mass. Eur. J. Pharm. Sci. 41 (3-4), 458–463. 10.1016/j.ejps.2010.07.015 - DOI - PubMed
LinkOut - more resources
Full Text Sources

