Causal Associations of Sleep Apnea With Alzheimer Disease and Cardiovascular Disease: A Bidirectional Mendelian Randomization Analysis
- PMID: 39258525
- PMCID: PMC11935638
- DOI: 10.1161/JAHA.123.033850
Causal Associations of Sleep Apnea With Alzheimer Disease and Cardiovascular Disease: A Bidirectional Mendelian Randomization Analysis
Abstract
Background: Sleep apnea (SA) has been linked to an increased risk of dementia in numerous observational studies; whether this is driven by neurodegenerative, vascular, or other mechanisms is not clear. We sought to examine the bidirectional causal relationships between SA, Alzheimer disease (AD), coronary artery disease (CAD), and ischemic stroke using Mendelian randomization.
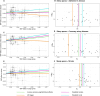
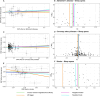
Methods and results: Using summary statistics from 4 recent, large genome-wide association studies of SA (n=523 366), AD (n=94 437), CAD (n=1 165 690), and stroke (n=1 308 460), we conducted bidirectional 2-sample Mendelian randomization analyses. Our primary analytic method was fixed-effects inverse variance-weighted (IVW) Mendelian randomization; diagnostics tests and sensitivity analyses were conducted to verify the robustness of the results. We identified a significant causal effect of SA on the risk of CAD (odds ratio [ORIVW]=1.35 per log-odds increase in SA liability [95% CI=1.25-1.47]) and stroke (ORIVW=1.13 [95% CI=1.01-1.25]). These associations were somewhat attenuated after excluding single-nucleotide polymorphisms associated with body mass index (ORIVW=1.26 [95% CI=1.15-1.39] for CAD risk; ORIVW=1.08 [95% CI=0.96-1.22] for stroke risk). SA was not causally associated with a higher risk of AD (ORIVW=1.14 [95% CI=0.91-1.43]). We did not find causal effects of AD, CAD, or stroke on risk of SA.
Conclusions: These results suggest that SA increased the risk of CAD, and the identified causal association with stroke risk may be confounded by body mass index. Moreover, no causal effect of SA on AD risk was found. Future studies are warranted to investigate cardiovascular pathways between sleep disorders, including SA, and dementia.
Keywords: Alzheimer disease; Mendelian randomization; cardiovascular diseases; causal inference; coronary artery disease; sleep apnea; stroke.
Figures
Update of
-
Causal Associations of Sleep Apnea with Alzheimer's Disease and Cardiovascular Disease: a Bidirectional Mendelian Randomization Analysis.medRxiv [Preprint]. 2023 Nov 22:2023.11.20.23298793. doi: 10.1101/2023.11.20.23298793. medRxiv. 2023. Update in: J Am Heart Assoc. 2024 Sep 17;13(18):e033850. doi: 10.1161/JAHA.123.033850. PMID: 38045267 Free PMC article. Updated. Preprint.
Comment in
-
Bidirectional Mendelian Randomization to Elucidate the Relationship Between Healthy Sleep, Brains, and Hearts.J Am Heart Assoc. 2024 Sep 17;13(18):e037394. doi: 10.1161/JAHA.124.037394. Epub 2024 Sep 11. J Am Heart Assoc. 2024. PMID: 39258560 Free PMC article. No abstract available.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

