Low-energy differential target multiplexed SCS derivative reduces pain and improves quality of life through 12 months in patients with chronic back and/or leg pain
- PMID: 39258956
- PMCID: PMC11680466
- DOI: 10.1111/papr.13407
Low-energy differential target multiplexed SCS derivative reduces pain and improves quality of life through 12 months in patients with chronic back and/or leg pain
Abstract
Introduction: Energy-reducing spinal cord stimulation (SCS) approaches have the potential to impact patient experience with rechargeable and non-rechargeable SCS devices through reducing device recharge time or enhancing device longevity. This prospective, multi-center study evaluated the safety, effectiveness, and actual energy usage of differential target multiplexed (DTM) endurance therapy, a reduced energy DTM SCS derivative.
Methods: Subjects who reported an overall pain visual analog score (VAS) of ≥6/10 cm and an Oswestry Disability Index score of 21-80 out of 100 at baseline with moderate to severe chronic, intractable back and/or leg pain were eligible. Evaluation visits occurred at 1, 3, 6, and 12 months post-device activation. The primary objective was to characterize change in overall pain intensity, as measured by VAS, from baseline to 3-month visit.
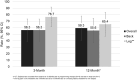
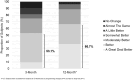
Results: Fifty-seven subjects enrolled at 12 US sites from November 2020 through June 2021, 35 were implanted with a rechargeable SCS device, and 27 completed the 12-month visit. Subjects experienced a 50.4% mean reduction in overall pain from baseline at the 3-month follow-up that was sustained through 12 months. Additional outcomes including changes in overall, back, and leg pain intensity, quality of life, disability, therapy satisfaction, safety, and current battery usage are shown through 12-month follow-up.
Conclusion: The use of DTM endurance SCS therapy in this study resulted in reductions in pain relief through 12 months, demonstrating that energy-reducing stimulation patterns can provide clinical benefit. Clinically effective, reduced energy SCS derivatives have the potential to impact patient experience through either reduced recharge requirements or increased device longevity.
Keywords: back pain; chronic pain; leg pain; reduced energy; spinal cord stimulation.
© 2024 Medtronic and The Author(s). Pain Practice published by Wiley Periodicals LLC on behalf of World Institute of Pain.
Conflict of interest statement
K. Amirdelfan reports consulting fees and research grant to institution from Medtronic. A. Calodney reports consulting fees to institution from PainTeq and TissueTech, payment/honoraria to institution from Medtronic, Stryker, Nevro, Boston Scientific, and Saluda. A. Calodney is an Editorial Board member of
Figures






References
-
- James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 disease and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Global Health Metr. 2018;392(10159):1789–1858. - PMC - PubMed
-
- Yong JR, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. Pain. 2022;163(2):e328–e332. 10/1087/j.pain.0000000000002291 - PubMed
-
- Kapural L, Yu C, Doust MW, Gliner BE, Vallejo R, Sitzman BT, et al. Novel 10‐kHz high‐frequency therapy (HF10 therapy) is superior to traditional low‐frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA‐RCT randomized controlled trial. Pain Med. 2015;123:851–860. 10/1097/ALN.000000000000774 - PubMed

