Liver adrenoceptor alpha-1b plays a key role in energy and glucose homeostasis in female mice
- PMID: 39259165
- PMCID: PMC11559639
- DOI: 10.1152/ajpendo.00153.2024
Liver adrenoceptor alpha-1b plays a key role in energy and glucose homeostasis in female mice
Abstract
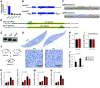
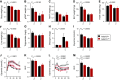
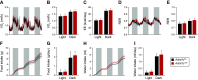
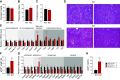
The liver plays a major role in glucose and lipid homeostasis and acts as a key organ in the pathophysiology of metabolic diseases. Intriguingly, increased sympathetic nervous system (SNS) activity to the liver has been associated with the development and progression of type 2 diabetes and obesity. However, the precise mechanisms by which the SNS regulates hepatic metabolism remain to be defined. Although liver α1-adrenoceptors were suggested to play a role in glucose homeostasis, the specific subtypes involved are unknown mainly because of the limitations of pharmacological tools. Here, we generated and validated a novel mouse model allowing tissue-specific deletion of α-1b adrenoceptor (Adra1b) in hepatocytes to investigate the role of liver ADRA1B in energy and glucose metabolism. We found that selective deletion of Adra1b in mouse liver has limited metabolic impact in lean mice. However, loss of Adra1b in hepatocytes exacerbated diet-induced obesity, insulin resistance, and glucose intolerance in female, but not in male mice. In obese females, this was accompanied by reduced hepatic gluconeogenic capacity and reprogramming of gonadal adipose tissue with hyperleptinemia. Our data highlight sex-dependent mechanisms by which the SNS regulates energy and glucose homeostasis through liver ADRA1B.NEW & NOTEWORTHY The sympathetic nervous system plays an important role in regulating hepatic physiology and metabolism. However, the identity of the adrenoceptors involved in these effects is still elusive. Using CRISPR-Cas9, we developed a novel transgenic tool to study the role of liver α-1b adrenoceptor (ADRA1B). We show that ADRA1B plays a key role in mediating the effects of the sympathetic nervous system on hepatic metabolism, particularly in female mice.
Keywords: adrenergic receptor; glucose metabolism; liver; sex difference; sympathetic nervous system.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



References
-
- International Diabetes Federation Diabetes Atlas. IDF Diabetes Atlas (10th ed.). Brussels: International Diabetes Federation, 2021, vol. 33.
-
- Mouchiroud M, Camiré E, Aldow M, Caron A, Jubinville E, Turcotte L, Kaci I, Beaulieu MJ, Roy C, Labbé SM, Varin TV, Gélinas Y, Lamothe J, Trottier J, Mitchell PL, Guénard F, Festuccia WT, Joubert P, Rose CF, Karvellas CJ, Barbier O, Morissette MC, Marette A, Laplante M. The hepatokine Tsukushi is released in response to NAFLD and impacts cholesterol homeostasis. JCI Insight 4: e129492, 2019. doi:10.1172/jci.insight.129492. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

