COVID-19 vaccines: anaphylaxis and anxiety : A case study from an allergy unit
- PMID: 39259224
- PMCID: PMC11534977
- DOI: 10.1007/s00508-024-02435-0
COVID-19 vaccines: anaphylaxis and anxiety : A case study from an allergy unit
Abstract
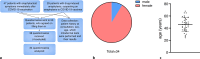
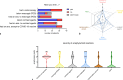
Background: Vaccination against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) was one crucial element to overcome the coronavirus disease 2019 (COVID-19) pandemic. Even though anaphylaxis to vaccines is rare, 47 patients came to the Allergy Unit at the University Hospital Graz, Austria, reporting immediate anaphylactoid symptoms after administration of COVID-19 vaccines. In addition, 29 patients with known drug-induced anaphylaxis wanted to be tested for a possible sensitization against COVID-19 vaccines or excipients, such as polyethylene glycol (PEG) or polysorbate 80 (PS80) before the first COVID-19 vaccination. Skin prick tests and intradermal tests were performed in all 76 patients, mostly using PEG 2000, and/or PS80. Skin prick tests with COVID-19 vaccines were performed depending on availability.
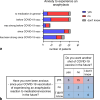
Objective: Our aim was to characterize this patient cohort in terms of patients' anaphylactoid responses, their willingness to future vaccinations against SARS-Cov‑2, and reasons for their decision.
Methods: We developed a questionnaire and analyzed 34 completed copies.
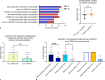
Results: Of the 47 patients with anaphylactoid reactions to COVID-19 vaccination, most were female (40 female/7 male). The skin tests, even when performed with the respective COVID-19 vaccine, were negative in all but one patient. Most patients who experienced anaphylactoid reactions after a COVID-19 vaccination, did not want another COVID-19 vaccination at the time of answering the questionnaire because of anxiety for another anaphylactoid response at the next shot. Premedication with antihistamines significantly lowered (n = 74 vaccinations) the severity of anaphylactoid responses after COVID-19 vaccinations.
Conclusion: Anxiety about another anaphylactoid episode hinders patients to be vaccinated against SARS-CoV‑2 again. Premedication with antihistamines and collaboration of allergologists with psychologists might lower the risk of an anaphylactic/anaphylactoid response as well anxiety in drug-induced anaphylactic patients.
Keywords: Anaphylactoid; Antihistamine; Intradermal test; Polyethylene glycol; Questionnaire.
© 2024. The Author(s).
Conflict of interest statement
A.R. Teufelberger, A.-R. Dan, L. Irmler, P. Wolf and B. Kränke declare that they have no competing interests. P. Wolf was supported by FWF Austrian Science Fund number W1241.
Figures
References
-
- Bruusgaard-Mouritsen MA, Johansen JD, Garvey LH. Allergy to polyethylene glycol has significant impact on daily life. Authorea; 2020. pp. 1–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

